 Biomarkers for neurodegenerative disorders are essential to facilitate disease diagnosis at early stages, monitor disease progression, and assess response to existing and future treatments[1]. A number of different biomarkers have been implicated in the development of neurodegenerative diseases, including Neurofilament light chain (NF-Light), amyloid-beta 40 (Aβ40), amyloid-beta 42 (Aβ42), glial fibrillary acidic protein (GFAP), Tau, and Ubiquitin C-terminal hydrolase L1 (UCH-L1)[1-6].
Biomarkers for neurodegenerative disorders are essential to facilitate disease diagnosis at early stages, monitor disease progression, and assess response to existing and future treatments[1]. A number of different biomarkers have been implicated in the development of neurodegenerative diseases, including Neurofilament light chain (NF-Light), amyloid-beta 40 (Aβ40), amyloid-beta 42 (Aβ42), glial fibrillary acidic protein (GFAP), Tau, and Ubiquitin C-terminal hydrolase L1 (UCH-L1)[1-6].
NF-Light is the most abundant intermediate filament protein in myelinated subcortical axons, which is important for the assembly and maintenance of the axonial cytoskeleton [2]. NF-Light levels are elevated in patients with various neurological diseases, including Alzheimer's, frontotemporal dementia, Huntington's disease, Amyotrophic lateral sclerosis, and multiple sclerosis[2, 7].
Aβ is a peptide produced as a result of the normal metabolism of the amyloid precursor protein (APP). However, in certain conditions, such as Alzheimer's disease, Ab can accumulate and form insoluble fibrils known as amyloid plaques [3]. Aβ40 and Aβ42 are the two most common forms of Aβ, and their relative levels have been implicated in the development and severity of Alzheimer's disease [3].
GFAP is a type of intermediate filament found in gilal cells. GFAP is the main structural protein of astrocytes and plays an important role in their function [4]. Mutations in the GFAP gene have been linked to several neurological disorders, including Alexander disease and Charcot-Marie-Tooth disease type 2 [4]. GFAP levels are known to be induced following brain damage or neurodegeneration [4].
Tau is a microtubule-associated protein that is found abundantly in the brain. It is involved in several neuronal fnctions, most notably in microtubule assembly and stablization [5]. In recent years, Tau has been implicated in a number of neurodegenerative diseases, including Alzheimer's disease, frontotemporal dementia, and Parkinson's disease[5, 8]. Alzheimer's disease is the most common form of dementia, and Tau has been shown to play a role in its pathogenesis. In Alheimer's disease, Tau becomes abnormally phosphorylated and forms neurofibrillary tangies, which are deposits of misfolded tau protein. These tangles are thought to disrupt microtubule function and lead to neuronal cell death [5, 8].
UCH-L1 is a ubiquitin C-terminal hydrolase that is responsible for hydrolyzing the final bond in monoubiquitinated proteins [6]. This protein plays an important role in proteasomal degradation and has also been implicated in neurodegenerative diseases such as Parkinson's and Alzheimer's [6]. UCH-L1 is also emerging as a promising neuro-derived biomarker for traumatic brain injury, ischemic and hypoxic stroke, pediatric hypoxic-ischemic encephalopathy, spinal cord injury, epileptic seizure, and cardiac arrest, and has strong potential as a robust and universal biomarker target for various forms of CNS injury [6, 9, 10].
To facilitate disease diagnosis, ideally in its earliest stages, monitor disease progression, and evaluate the effects of existing and future treatments, biomarkers for neurodegenerative disorders are essential [1]. In conclusion, the use of NF-Light in conjunction with other biomarkers can provide a more comprehensive approach to neurodegenerative disease diagnosis [2].
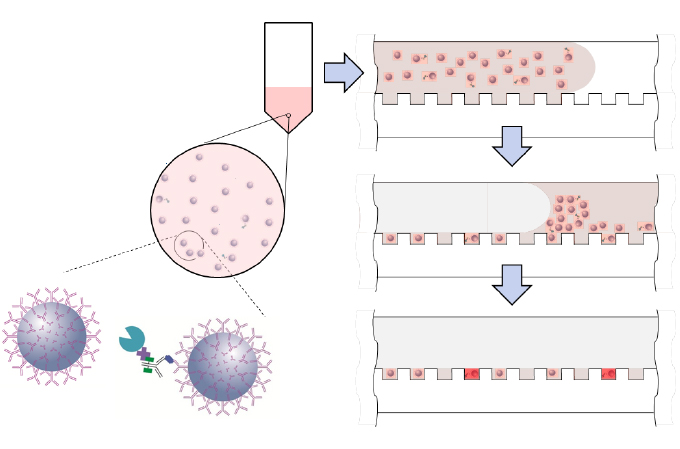 The Single Molecule Array (SIMOA) assay panels can simultaneously analyze many of these biomarkers at sub femtomolar levels. The Neurology 4-plex E panel, for example, can measure NF-Light, Amyloid-beta 40, Amyloid-beta 42, and GFAP together. The Neurology 4-Plex A measures NF-Light, Tau, GFAP, and UCH-L1 simultaneously. Neurology 3-Plex A can measure Amyloid-beta 40 and 42 as well as total Tau proteins, while Neurology 2-Plex B is designed to measure NF-Light and total Tau [11].
The Single Molecule Array (SIMOA) assay panels can simultaneously analyze many of these biomarkers at sub femtomolar levels. The Neurology 4-plex E panel, for example, can measure NF-Light, Amyloid-beta 40, Amyloid-beta 42, and GFAP together. The Neurology 4-Plex A measures NF-Light, Tau, GFAP, and UCH-L1 simultaneously. Neurology 3-Plex A can measure Amyloid-beta 40 and 42 as well as total Tau proteins, while Neurology 2-Plex B is designed to measure NF-Light and total Tau [11].
If you are interested in learning more about our multi-biomarker detection assay services, please connect with our scientific team. We would be happy to discuss your specific needs and how we can help you obtain the accurate measurements you need.
References
[1] Hampel, H., O'Bryant, S. E., Durrleman, S., Younesi, E., Rojkova, K., Escott-Price, V., ... & Koronyo, Y. (2017). A precision medicine initiative for Alzheimer's disease: the road ahead to biomarker-guided integrative disease modeling. Climacteric, 20(2), 107-118.
[2] Khalil, M., Teunissen, C. E., Otto, M., Piehl, F., Sormani, M. P., Gattringer, T., ... & Al-Sharafi, M. A. (2020). Neurofilaments as biomarkers in neurological disorders. Nature Reviews Neurology, 16(12), 690-704.
[3] Bateman, R. J., Xiong, C., Benzinger, T. L., Fagan, A. M., Goate, A., Fox, N. C., ... & Allegri, R. (2012). Clinical and biomarker changes in dominantly inherited Alzheimer's disease. New England Journal of Medicine, 367(9), 795-804.
[4] Brenner, M., Johnson, A. B., Boespflug-Tanguy, O., Rodriguez, D., Goldman, J. E., & Messing, A. (2001). Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nature Genetics, 27(1), 117-120.
[5] Zhang, B., Higginbotham, L., Smith, A. D., Gleichman, A. J., Shu, H., & Diamantopoulos, G. N. (2018). Tau proteins: normal function and role in neurodegenerative diseases. In Neurodegenerative Diseases: Pathology, Mechanisms, and Potential Therapeutic Targets (pp. 1-34). Springer.
[6] Mollenhauer, B., Zhang, J., & Shaw, L. M. (2011). Alzheimer's Disease Neuroimaging Initiative. Stability of CSF biomarkers in Alzheimer's disease. Alzheimer's & Dementia, 7(4), 386-395.
[7] Skillbäck, T., Zetterberg, H., & Blennow, K. (2017). Neurofilament protein in cerebrospinal fluid: a marker of white matter changes. Journal of Neuroscience Research, 95(1-2), 157-162.
[8] Ittner, L. M., & Gotz, J. (2011). Amyloid-β and tau--a toxic pas de deux in Alzheimer's disease. Nature Reviews Neuroscience, 12(2), 67-72.
[9] Liliang, P. C., Liang, C. L., Weng, H. C., Lu, K., Wang, K. W., Chen, H. J., ... & Yang, C. H. (2010). Tau proteins in serum predict outcome after severe traumatic brain injury. Journal ofNeurosurgery, 112(5), 1214-1220.
[10] Mondello, S., Buki, A., Barzo, P., Randall, J., Provuncher, G., Hanlon, D., ... & Gabrielli, A. (2012). CSF and plasma amyloid-β temporal profiles and relationships with neurological status and mortality after severe traumatic brain injury. Scientific Reports, 2(1), 1-8.
[11] Quanterix. (n.d.). Simoa Neurology Assays. Retrieved from https://www.quanterix.com/neurology-assays
