Serum Soluble IFNAR2 Levels In Autoimmune Diseases Suggest That Chronic IFN Signaling Leads To Elevated Level sIFNAR2
Abstract:
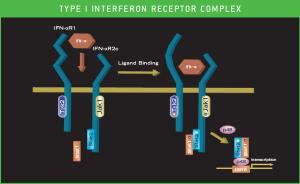
The Type I Interferon (IFN) receptor consists of a heterodimer of chain 1 (IFNAR1) which is required for signaling and chain 2 (IFNAR2) which binds tightly to IFN. Soluble forms of the IFNAR2 can be produced either by proteolytic cleavage or production of an alternatively splice transcript. Soluble IFNAR2 can be found in serum and urine however its role and regulation are poorly understood. Studies have suggested that sIFNAR2 is elevated in MS patients, advanced cancer patients and hepatitis C infected patients. Using a newly developed ELISA for sIFNAR2 we have examined commercially sourced serum/plasma samples from autoimmune patients in comparison to normal donors. In comparison to 35 normal donors MS patients on IFN therapy(n=29) had significantly (p=0.0005) increased levels of sIFNAR2 while those on other therapies (n=24) were not different from the normal population but were significantly different than those MS patients on IFN therapy (p=<0.0001). sIFNAR2 levels showed no correlation with treatments such as vitamin-D, copaxone or natalizumab. Systemic Lupus patients (n=67) had elevated sIFNAR2 (p=0.009) while rheumatoid arthritis (n=16) were not significantly different from normal Sjogren’s patients (n=11) and scleroderma patients (n=10) Although evidence of Type I IFN activation has been observed in RA, Sjogren’s and Scleroderma patients the intensity is thought to be less than for MS patients on IFN-therapy and Systemic Lupus. These data suggest a model where long term IFN signaling above a certain threshold leads to elevated sIFNAR2.

Related Article
Validation Of a Highly Sensitive Immunoassay For The Quantitation Of Interferon Beta In Autoimmune Sera
Read Article