Treating Multiple Sclerosis with Interferon-Beta
Multiple sclerosis (MS) is an inflammatory disease of the CNS. In the last decade, there has been an increase in the number of studies trying to determine the immune mechanisms that mediate tissue damage in MS (Javed and Reder, 2005; Weiner, 2008). IFN-β is effective for treating Multiple Sclerosis (MS); however the mechanism by which the therapeutic effect takes place remains somewhat elusive to date (Pozzilli and Prosperini, 2008). It is thought that among other pathogenic targets, IFN-β down-regulates T-cell activation by altering the expression of proteins involved in antigen presentation, and studies indicate that it also promotes the differentiation of activated T cells away from a T-helper-type 1 Th1 response (pro-inflammatory) and towards a Th2 response (anti-inflammatory). Accordingly, IFN-β decreases the expression of IL-12 and promotes the expression of anti-inflammatory cytokines like IL-27 by macrophages. (Baccala et al., 2005).
Figure 1.IFN-β diminishes the ability of activated T cells to cross the blood-brain barrier
and enter the central nervous system parenchyma
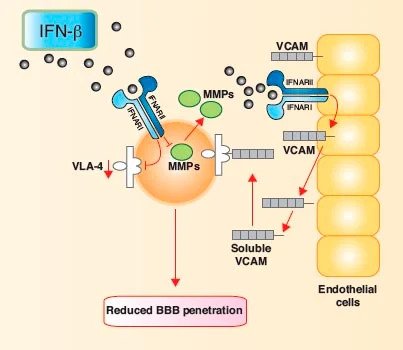
Systemic administration of IFN-β has been shown to slow MS disease progression, reduce relapse rate, and decrease the number of CNS lesions. Although the signaling pathways remain to be deduced, results clearly implicate the involvement of IFN-β in regulating the inflammatory response in the CNS. Other autoimmune diseases similar to MS that are characterized by a T-lymphocyte delayed-type hypersensitivity response include rheumatoid arthritis (RA) and Type I diabetes mellitus (DM). The diseases are caused by the similar immune deregulation found in the synovial membrane lining the non-cartilaginous surfaces of the joints, and the b islet cells of the pancreas, respectively. FDA-approved use of injectable IFN-β 1b (Betaseron®, Bayer) (Lin, 1998) to treat MS has been shown to decrease neuronal lesions by 80%, whereas Phase I trials using intramuscular treatment of IFN-α2a (Roferon A, Roche) yields a reduction in the clinical signs of relapse-remitting MS. Moreover, Phase I clinical trials using ingested recombinant human IFN-α2 showed a 30% preservation of b cell function in Type 1 diabetes, as well as a 20% reduction in the signs of symptoms associated with painful and swollen joints in RA (Brod, 2002). Crohn’s disease is another more recently described autoimmune condition which is also thought to arise primarily due to inappropriate chronic T-lymphocyte activation, with tissue damage induced by secondary macrophage activation. The prospect that Crohn’s disease is a form of T-helper type 1 (Th1) cell-dominant autoimmune disease is gaining acceptance, with support from the current use of immunosuppressants, and the possibility of IFN-β utilization as a therapeutics becoming much more probable (Rossi et al., 2009).
IFN-β treatment of MS patients restores immune dysfunction to a degree, but not completely. The incomplete resolution of immune dysfunction by IFNs partly explains their significant, but modest therapeutic effects. This also suggests that there are immune mechanisms in MS and probably other autoimmune diseases that are resistant to IFN therapy. In all autoimmune diseases, abnormalities may exist at several points along the IFN signaling pathway, including molecular defects in the IFN signaling pathways. Currently, many studies are focusing on evaluating ways of differentiating and modulating IFN effects. IFN-β was the first agent to show substantial clinical benefits in the treatment of MS. Many years of experience with these molecules has confirmed clinical efficacy over extended time. In the near future, IFN-based therapeutics will likely continue to play a significant role in treating a variety of autoimmune disorders.
References
-
Baccala, R., D.H. , and A.N. Theophelopoulos, Interferons as pathogenic effectors in autoimmunity. Immunol Rev, 2005. 204(1):9-26.
-
Brod, S.A., Ingested type I interferon: A potential treatment for autoimmunity. J. Interferon Cytokine Res, 2002. 22:1153-66.
-
Javed, A. and A.T. Reder, Therapeutic role of beta-interferons in multiple sclerosis. Pharmacol Ther, 2005. 110(1):35-56.
-
Lin, L., Betaseron. Dev Biol Stand, 1998. 96:97-104.
-
Pozzilli, C. and L. Prosperini, Clinical markers of therapeutic response to disease modifying drugs. Neurol Sci, 2008. 29(Suppl 2):211-3.
-
Rossi, C.P., et al., IFN-beta1 for the maintenance of remission in patients with Crohn’s disease: results of a phase II dose-finding study. BMC Gastroenterology, 2009. 9(22).
-
Weiner, H.L., A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J. Neurol, 2008. 255(Suppl 1):3-11
Treating Multiple Sclerosis with Interferon-Beta
Multiple sclerosis (MS) is an inflammatory disease of the CNS. In the last decade, there has been an increase in the number of studies trying to determine the immune mechanisms that mediate tissue damage in MS (Javed and Reder, 2005; Weiner, 2008). IFN-β is effective for treating Multiple Sclerosis (MS); however the mechanism by which the therapeutic effect takes place remains somewhat elusive to date (Pozzilli and Prosperini, 2008). It is thought that among other pathogenic targets, IFN-β down-regulates T-cell activation by altering the expression of proteins involved in antigen presentation, and studies indicate that it also promotes the differentiation of activated T cells away from a T-helper-type 1 Th1 response (pro-inflammatory) and towards a Th2 response (anti-inflammatory). Accordingly, IFN-β decreases the expression of IL-12 and promotes the expression of anti-inflammatory cytokines like IL-27 by macrophages. (Baccala et al., 2005).

Systemic administration of IFN-β has been shown to slow MS disease progression, reduce relapse rate, and decrease the number of CNS lesions. Although the signaling pathways remain to be deduced, results clearly implicate the involvement of IFN-β in regulating the inflammatory response in the CNS. Other autoimmune diseases similar to MS that are characterized by a T-lymphocyte delayed-type hypersensitivity response include rheumatoid arthritis (RA) and Type I diabetes mellitus (DM). The diseases are caused by the similar immune deregulation found in the synovial membrane lining the non-cartilaginous surfaces of the joints, and the b islet cells of the pancreas, respectively. FDA-approved use of injectable IFN-β 1b (Betaseron®, Bayer) (Lin, 1998) to treat MS has been shown to decrease neuronal lesions by 80%, whereas Phase I trials using intramuscular treatment of IFN-α2a (Roferon A, Roche) yields a reduction in the clinical signs of relapse-remitting MS. Moreover, Phase I clinical trials using ingested recombinant human IFN-α2 showed a 30% preservation of b cell function in Type 1 diabetes, as well as a 20% reduction in the signs of symptoms associated with painful and swollen joints in RA (Brod, 2002). Crohn’s disease is another more recently described autoimmune condition which is also thought to arise primarily due to inappropriate chronic T-lymphocyte activation, with tissue damage induced by secondary macrophage activation. The prospect that Crohn’s disease is a form of T-helper type 1 (Th1) cell-dominant autoimmune disease is gaining acceptance, with support from the current use of immunosuppressants, and the possibility of IFN-β utilization as a therapeutics becoming much more probable (Rossi et al., 2009).
IFN-β treatment of MS patients restores immune dysfunction to a degree, but not completely. The incomplete resolution of immune dysfunction by IFNs partly explains their significant, but modest therapeutic effects. This also suggests that there are immune mechanisms in MS and probably other autoimmune diseases that are resistant to IFN therapy. In all autoimmune diseases, abnormalities may exist at several points along the IFN signaling pathway, including molecular defects in the IFN signaling pathways. Currently, many studies are focusing on evaluating ways of differentiating and modulating IFN effects. IFN-β was the first agent to show substantial clinical benefits in the treatment of MS. Many years of experience with these molecules has confirmed clinical efficacy over extended time. In the near future, IFN-based therapeutics will likely continue to play a significant role in treating a variety of autoimmune disorders.
References
-
Baccala, R., D.H. , and A.N. Theophelopoulos, Interferons as pathogenic effectors in autoimmunity. Immunol Rev, 2005. 204(1):9-26.
-
Brod, S.A., Ingested type I interferon: A potential treatment for autoimmunity. J. Interferon Cytokine Res, 2002. 22:1153-66.
-
Javed, A. and A.T. Reder, Therapeutic role of beta-interferons in multiple sclerosis. Pharmacol Ther, 2005. 110(1):35-56.
-
Lin, L., Betaseron. Dev Biol Stand, 1998. 96:97-104.
-
Pozzilli, C. and L. Prosperini, Clinical markers of therapeutic response to disease modifying drugs. Neurol Sci, 2008. 29(Suppl 2):211-3.
-
Rossi, C.P., et al., IFN-beta1 for the maintenance of remission in patients with Crohn’s disease: results of a phase II dose-finding study. BMC Gastroenterology, 2009. 9(22).
-
Weiner, H.L., A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J. Neurol, 2008. 255(Suppl 1):3-11
Related Article
Interferon-beta therapy increases concentration of soluble Interferon alpha/beta receptor in Multiple Sclerosis patient serum
Read Article
