iLite Human Type I IFN Responsive Cells
Catalog Number: 51100
Reporter gene cells for Human Type 1 Interferon bioactivity measurement. Indirectly measure Neutralizing Antibodies (NAbs) to IFN-Alpha or IFN-Beta
$1,245.00
Product Info
Suggested Applications*
*Please note that these applications are presented for suggested use only and have not been fully evaluated by PBL
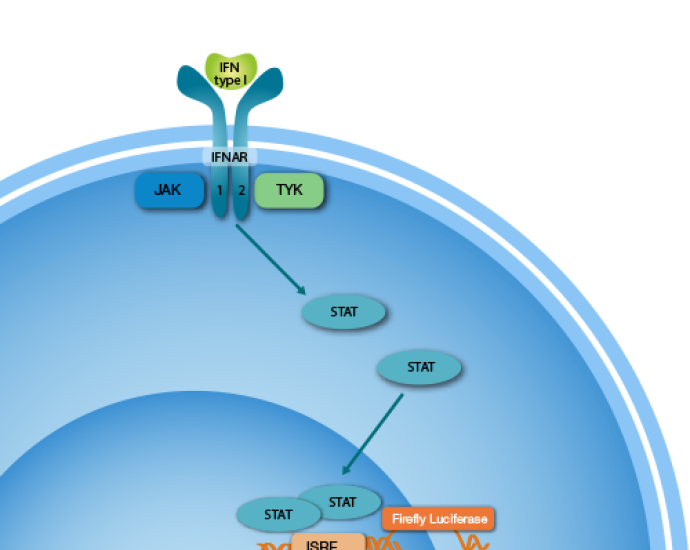
The iLite Human Type I Interferon Responsive Cells are engineered cells optimized to express Firefly Luciferase under the control of an IFN a/b responsive promoter. IFN-Alpha or IFN-Beta binds to the IFN a/b receptor on the cell surface and activates the IFN a/b regulated Firefly Luciferase reporter gene construct.
These growth-arrested cells are sensitive to human Type I IFN. The cells can be used in a number of different assays for measuring the bioactivity of Type I IFN or NAbs to IFN-Beta or NAbs to IFN-Alpha.
- Provided as assay-ready frozen cells
- The cell line is highly specific for its target due to cell engineering using target-specific reporter gene constructs
Specifications
| Formulation | Supplied frozen diluted in RPMI 1640 with 20% heat inactivated fetal bovine serum (FBS), 10% glycerol and 2.5% dimethyl sulfoxide (DMSO) |
|---|---|
| Antigen | Type 1 Interferon (Type I IFN) |
| Source | Engineered cells |
| Storage | After receipt, store the product at -80 ºC (do not store at any other temperature). Thawed cells should be used within 30 minutes. |
| Synonyms | Type I IFN, Type 1 IFN, Type I Interferon, Type 1 Interferon, Human, IFN-alpha, IFN-beta, IFNa, IFNb, Interferon alpha, Interferon beta, iLite |
Tech Info & Data
Application Notes (by request)
- Quantification of Interferon a or b using iLite Type 1 IFN Assay Ready Cells
- Measuring neutralizing antibodies to IFNa/b using iLite Type 1 IFN Assay Ready Cells
Background
IFN-Alpha has been widely used to treat chronic viral hepatitis and a wide variety of malignant diseases, including hairy cell leukemia, basal cell carcinoma, chronic myeloid leukemia, and cutaneous T-cell lymphoma. Several different recombinant preparations of IFNa are available commercially; the most commonly used formulations include IFNa2a and IFNa2b. A number of studies have shown that development of anti-IFNa neutralizing antibodies (NAbs) is correlated with a loss of IFNa treatment efficacy.
IFN-Beta is well established as first line therapy in relapsing/remitting multiple sclerosis. The occurrence of NAbs and binding antibodies (BAbs) to IFNb has been widely reported. Subjects with NAbs have shown reduced response to treatment with IFNb, having higher relapse rates, increased MRI activity and higher risk of disease progression. The frequencies and titers of NAbs vary depending on the preparation used, dose and frequency of administration and also the assay used to quantify them.
Citations
4 Citations:
- Pogue, Sarah, et al. (2016). Targeting Attenuated Interferon-α to Myeloma Cells with a CD38 Antibody Induces Potent Tumor Regression with Reduced Off-Target Activity. PLOS One, 20 pgs. PMID: 27611189. (link)
- Hermanrud, et al. (2016). Development and Validation of Cell-Based Luciferase Reporter Gene Assays for Measuring Neutralizing Anti-Drug Antibodies Against Interferon Beta. Journal of Immunology, 8 pgs. PMID: 26779831. (link)
- Grossberg, et al. (2001). The Neutralization of Interferons by Antibody: II. Neutralizing Antibody Unitage and its Relationship to Bioassay Sensitivity, the Tenfold Reduction Unit. Journal of Interferon & Cytokine Research, 12 pgs. PMID: 11576468. (link)
- Grossberg, et al. (2001). The Neutralization of Interferons by Antibody: I. Quantitative and Theoretical Analyses of the Neutralization Reaction in Different Bioassay Systems. Journal of Interferon & Cytokine Research, 13 pgs. PMID: 11576467. (link)