Methods:
Singulex® IL6, IL17F, IL17A and IL1β assay kits were evaluated. Lower limits of quantitation (LLOQ) and limits of detection (LOD) were calculated from standard curves. Quantitation of endogenous levels in commercially sourced normal human sera and plasma was performed. Inter-assay precision of endogenous analytes was determined using distinct runs. Specificity was assessed by signal reduction using polyclonal antibodies (PAbs) or exchanging capture microparticles for different analytes. Linearity of dilution and spike recovery was assessed in normal human serum and/or plasma.
Results:
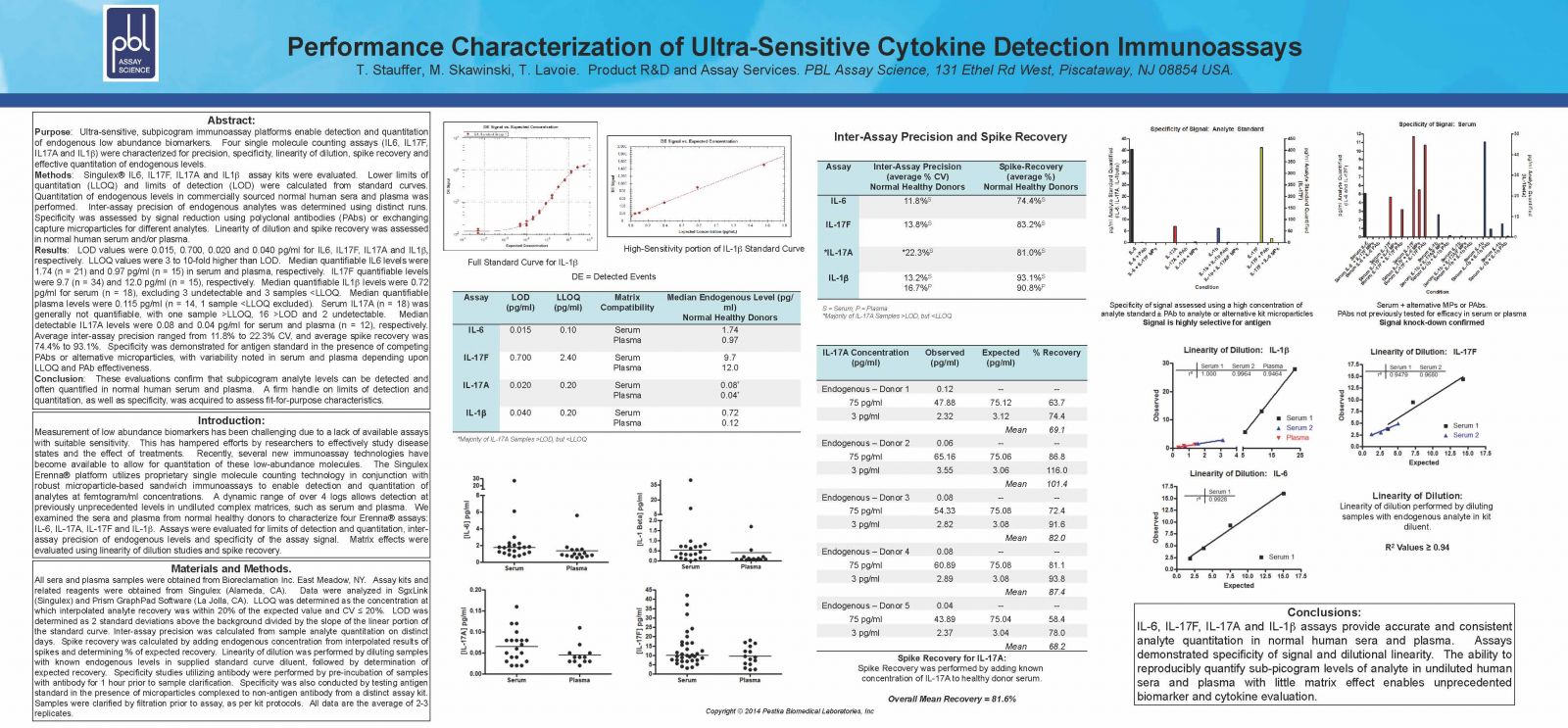
LOD values were 0.015, 0.700, 0.020 and 0.040 pg/ml for IL6, IL17F, IL17A and IL1 respectively. LLOQ values were 3 to 10-fold higher than LOD.Median quantifiable IL6 levels were 1.74 (n = 21) and 0.97 pg/ml (n = 15) in serum and plasma, respectively. IL17F quantifiable levels were 9.7 (n = 34) and 12.0 pg/ml (n = 15), respectively. Median quantifiable IL1 levels were 0.72 pg/ml for serum (n = 18), excluding 3 undetectable and 3 samples <LLOQ. Median quantifiable plasma levels were 0.115pg/ml (n = 14, 1 sample <LLOQ excluded). Serum IL17A (n = 18) was generally not quantifiable, with one sample >LLOQ, 16 >LOD and 2 undetectable. Median detectable IL17A levels were 0.08 and 0.04 pg/ml for serum and plasma (n=12), respectively. Average inter-assay precision ranged from 11.8% to 22.3% CV, and average spike recovery was 74.4% to 93.1%. Specificity was demonstrated for antigen standard in the presence of competing PAbs or alternative microparticles, with variability noted in serum and plasma depending upon LLOQ and PAb effectiveness.
Conclusion:
These evaluations confirm that subpicogram analyte levels can be detected and often quantified in normal human serum and plasma. A firm handle on limits of detection and quantitation, as well as specificity, was acquired to assess fit-for-purpose characteristics.
Methods:
Singulex® IL6, IL17F, IL17A and IL1β assay kits were evaluated. Lower limits of quantitation (LLOQ) and limits of detection (LOD) were calculated from standard curves. Quantitation of endogenous levels in commercially sourced normal human sera and plasma was performed. Inter-assay precision of endogenous analytes was determined using distinct runs. Specificity was assessed by signal reduction using polyclonal antibodies (PAbs) or exchanging capture microparticles for different analytes. Linearity of dilution and spike recovery was assessed in normal human serum and/or plasma.
Results:
LOD values were 0.015, 0.700, 0.020 and 0.040 pg/ml for IL6, IL17F, IL17A and IL1 respectively. LLOQ values were 3 to 10-fold higher than LOD.Median quantifiable IL6 levels were 1.74 (n = 21) and 0.97 pg/ml (n = 15) in serum and plasma, respectively. IL17F quantifiable levels were 9.7 (n = 34) and 12.0 pg/ml (n = 15), respectively. Median quantifiable IL1 levels were 0.72 pg/ml for serum (n = 18), excluding 3 undetectable and 3 samples <LLOQ. Median quantifiable plasma levels were 0.115pg/ml (n = 14, 1 sample <LLOQ excluded). Serum IL17A (n = 18) was generally not quantifiable, with one sample >LLOQ, 16 >LOD and 2 undetectable. Median detectable IL17A levels were 0.08 and 0.04 pg/ml for serum and plasma (n=12), respectively. Average inter-assay precision ranged from 11.8% to 22.3% CV, and average spike recovery was 74.4% to 93.1%. Specificity was demonstrated for antigen standard in the presence of competing PAbs or alternative microparticles, with variability noted in serum and plasma depending upon LLOQ and PAb effectiveness.
Conclusion:
These evaluations confirm that subpicogram analyte levels can be detected and often quantified in normal human serum and plasma. A firm handle on limits of detection and quantitation, as well as specificity, was acquired to assess fit-for-purpose characteristics.