MilliporeSigma Single Molecule Counting (SMCxPRO® & Erenna®)
-
Cytokine analysis utilizing Single Molecule Counting technology enables sub-pg/ml sensitivity for accurate measurement of low abundance biomarkers in normal or disease sera/plasma
-
Minimal sample dilution requirement with 4-Log+ dynamic range
-
High precision cytokine immunoassays provide reproducible data
-
Extensive analyte menu of over 40 different cytokine assays covering several disease areas including IFN-γ, IL-1a, IL-1b, IL-4, IL-6, IL-8, IL-10, IL-13, IL-15, IL-17A, IL-17F, IL-17 heterodimer, IL-23, GLP-1, and TNF-α
- See below for representative data
Employing MilliporeSigma's Single Molecule Counting (SMC) technology with robust microparticle-based SMCxPRO® & Erenna® immunoassays, PBL’s cytokine detection services can provide scientists with sub-pg/ml level measurements of low-abundance analytes in healthy or disease sera and plasma.

Request an Assay Services Quote
Key Features offered by Single Molecule Counting Technology on SMCxPRO and Erenna Platforms:
-
Enables sub-pg/ml sensitivity for accurate measurement of low abundance biomarkers in serum, plasma or other complex matrices
-
Requires minimal sample dilution with a 4-Log+ dynamic range
-
Provides high precision and reproducible data
-
Extensive analyte menu of over 30 different cytokine assays covering several disease areas
Powered by SMCxPRO® & Erenna® Immunoassay Systems, MilliporeSigma Inc., Burlington, MA, USA.
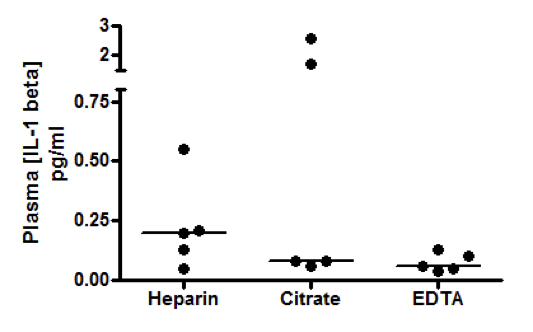
2 Citations:
- Passeron, T. et al., (2023), "A Phase IIIb, Multicentre, Interventional, Randomised, Placebo-Controlled Clinical Trial Investigating the Efficacy and Safety of Guselkumab for the Treatment of Nonpustular Palmoplanter Psoriasis (G-PLUS)", Dermatologic Therapy, DOI: 10.1155/2023/9967747 (link)
- Morita, A. et al., (2022), "Effect of guselkumab on serum biomarkers in Japanese palmoplanter pustulosis patients in randomized phase 3 study", JEADV Clinical Practice, DOI: 10.1002/jvc2.73 (link)
