Objective
Explore ranges of IL-22 serum concentrations to differentiate between healthy donors (HD) and select autoimmune disease patients with a simple-to-use, high sensitivity ELISA.
Methods
A commercial sandwich ELISA (PBL Assay Science, 41701-1) quantifying human IL-22 was characterized and used to assess IL-22 concentration in sera from HD and patients exhibiting one of several autoimmune patients.
Results
This high sensitivity ELISA,m having a manufacturer-stated LLOQ of 0.78 pg/ml, exhibit good assay precision, dilutional linearity, and spike recovery using HD serum. Parallelism was demonstrated using serum samples from four AD patients and three HD. Addition of a 10-to-100-fold mass excess of exgenous recombinant IL-22BP reduced the ability of the assay to quantify IL-22 suggesting that at least one antigenic region recognized by ELISA antibodies may be sterically hindered by IL-22BP binding to IL-22. However, such high levels of 10 and 100 ng/ml of IL-22BP are unlikely to be achieved in vivo, and therefore may not adversely affect quantitation of physiological levels of IL-22 protein by this assay.
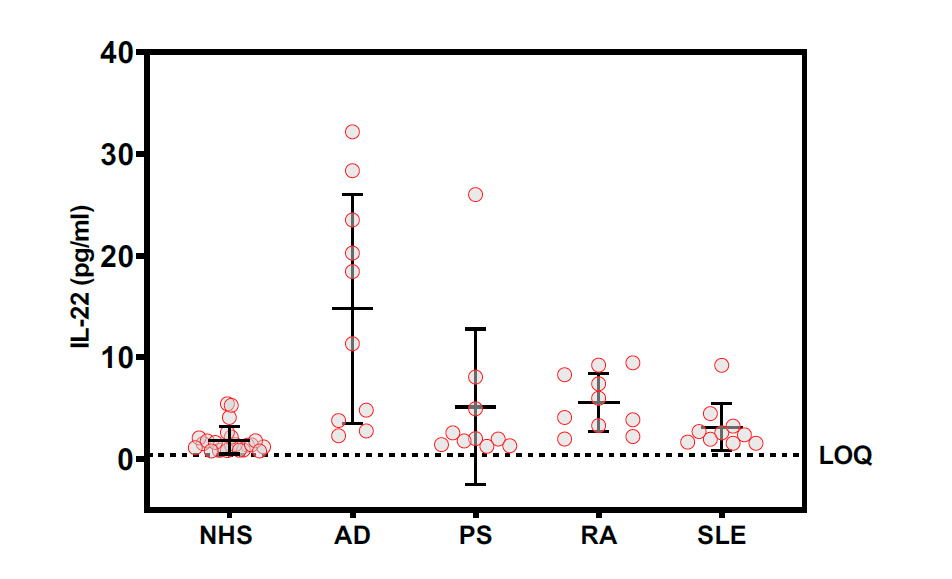
Commercially sourced HD sera (NHS) concentrations of IL-22 exceeded the assay LLOQ in 23 of 24 samples (96%) with a mean IL-22 concentration of 1.84 +/- 1.33 pg/ml. Commercial sera from AD (n=10, 100%). PS (n=10, 100%), RA (n=10, 100%), SLE (n=10, 100%) patients were similarly analyzed and yielded mean levels of 14.8 +/- 11.2, 5.1 +/- 0.91, and 3.1 +/- 0.74 pg/ml, respectively.
Conclusions
IL-22 measured in AD (and PS) sera exhibit sufficiently broad ranges of concentrations such that statistical differences between AD and all other groups were not readily achievable with this number of samples. Notably, these commercially obtained serum samples were not controlled for overall severity of any disease at the time of sample collection, Sample sets were randomly obtained from a population of individuals self-identifying as AD sufferers, as were the other patient samples. With mean serum IL-22 in AD appearing to trend as substantially elevated, an opportunity exists to explore IL-22 trends along with other biomarkers in clinically identified and possibly symptom-stratified populations of AD patients. Should this trend toward IL-22 elevation be fully substantiated in AD, therapeutic stratification and precision medicine approaches may be conceivable based on serum IL-22 protein concentrations, supporting other investigators' earlier work examining IL-22 mRNA(9). The baseline readability of endogenous IL-22 in HD sera using this ELISA is also highly advantageous.
Ketwords
Atopic dermatitis, IL-22, ELISA, autoimmune, basal level
References
1. Dumoutier L., Van Roost E., Michaux L., Renald J.C., (2000) IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun., 1:488-94, DOI: 101038/sj.gene.6363716
2. Dudakov J.A., Hanash A.M., van den Brink, M.R.M., (2015) Interleukin-22: Immunobiology and Pathology. Ann. Rev. Immunol., 33:747-785, DOI: 10.1146/annurevimmunol-032414-112123
3. Arshad T., Mansur F., Palek R., Manzoor S and Liska V., (2020), A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol., 11:2148, DOI: 10.3389/fimmu.2020.02148
4. Eyerich K., Dimartino V., Cavani A., (2017), IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol., 47(4):607-614, DOI: 10.1002/eji.201646723
5. Li L.J., Gong C., Zhao M.H. Feng B.S. (2014), Role of interleukin-22 in inflammatory bowel disease. World J. Gastroenterol., 20(48):18177-18188, DOI: 10.3748/wjg.v20.i48.18177
6. Brand S., Beigel F., Olszak T., Zitzmann K., Eichhorst S.T., Otte J.M., Diepolder H., Marquardt A., Jagla W., Popp A., Leclair S., Herrmann K., Seiderer J., Ochsenkuhn T., Goke B., Auernhammer C.J., Dambacher J., (2006), IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration, Am. J. Physiol. Gastrointest Liver Physiol., 290:G827-838, DOI: 10.1152/ajpgi.00513.2005
7. Aujla S.J., Chan Y.R., Zheng M., Fei M., Askev D.J., Pociask D.A., Reinhart T.A., McAllister F., Edeal J., Gaus K., Husain S., Kreindler J.L., Dubin P.J., Pilewski J.M., Myerburg M.M., Mason C.A., Iwakura Y., Kolls J.K., (2008), IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia, Nat. Med., 14:275-281, DOI: 10.1038/nm1710
8. Eyerich S., Traidl-Hoffmann C., Behrendt H., Cavani A., Schmidt-Weber C.B., Ring J., Eyerich K., (2017), Novel key cytokines in allergy: IL-17, IL-22. Allergologie Select., 1(1):71-76
9. Brunner P.M., Pavel A.B., Khattri S., Leonard A., Malik K., Rose S., Jim On S., Vekaria A.S., Traidl-Hoffmann C., Singer G.K., Baum D., Gilleaudeau P., Sullivan-Whalen M., Fuentes-Duculan J., Li X., Zheng X., Estrada Y., Garcet S., Wen H.C., Gonzalez J., Coats I., Cueto I., Neumann A.U., Lebwohl M.G., Krueger J.G., Guttman-Yassky E., (2019), Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab
10. Miyagawa I., Nakayamada S., Ueno M., et al., (2022), Impact of serum interleukin-22 as a biomarker for the differential use of molecular targeted drugs in psoriatic arthritis: a retrospective study., Arthritis Res. Ther., 24:86, DOI: 10.1186/s13075-022-02771-4
11. Lavoie T.N., Stewart C.M., Berg K.M., Li Y., Nguyen C.Q., (2011), Expression of interleukin-22 in Sjorgen's syndrome: significant correlation with disease parameters., Scan. J. Immunol., 74(4):377-381, DOI: 10.1111/j.1365-3085.2011.02583.x
12. M Martin J.C., Beriou G., Heslan M., Chauvin C., Utriainen L., Aumeunier A., Scott C.L., Mowat A., Cerovic V., Houston S.A., Leboeuf M., (2014), Interleukin-22 binding protein cells and is strongly induced by retinoic acid., Mucosal Immunol. 7(1):101-113, DOI: 10.1038/mi.2013.28
Objective
Explore ranges of IL-22 serum concentrations to differentiate between healthy donors (HD) and select autoimmune disease patients with a simple-to-use, high sensitivity ELISA.
Methods
A commercial sandwich ELISA (PBL Assay Science, 41701-1) quantifying human IL-22 was characterized and used to assess IL-22 concentration in sera from HD and patients exhibiting one of several autoimmune patients.
Results
This high sensitivity ELISA,m having a manufacturer-stated LLOQ of 0.78 pg/ml, exhibit good assay precision, dilutional linearity, and spike recovery using HD serum. Parallelism was demonstrated using serum samples from four AD patients and three HD. Addition of a 10-to-100-fold mass excess of exgenous recombinant IL-22BP reduced the ability of the assay to quantify IL-22 suggesting that at least one antigenic region recognized by ELISA antibodies may be sterically hindered by IL-22BP binding to IL-22. However, such high levels of 10 and 100 ng/ml of IL-22BP are unlikely to be achieved in vivo, and therefore may not adversely affect quantitation of physiological levels of IL-22 protein by this assay.

Commercially sourced HD sera (NHS) concentrations of IL-22 exceeded the assay LLOQ in 23 of 24 samples (96%) with a mean IL-22 concentration of 1.84 +/- 1.33 pg/ml. Commercial sera from AD (n=10, 100%). PS (n=10, 100%), RA (n=10, 100%), SLE (n=10, 100%) patients were similarly analyzed and yielded mean levels of 14.8 +/- 11.2, 5.1 +/- 0.91, and 3.1 +/- 0.74 pg/ml, respectively.
Conclusions
IL-22 measured in AD (and PS) sera exhibit sufficiently broad ranges of concentrations such that statistical differences between AD and all other groups were not readily achievable with this number of samples. Notably, these commercially obtained serum samples were not controlled for overall severity of any disease at the time of sample collection, Sample sets were randomly obtained from a population of individuals self-identifying as AD sufferers, as were the other patient samples. With mean serum IL-22 in AD appearing to trend as substantially elevated, an opportunity exists to explore IL-22 trends along with other biomarkers in clinically identified and possibly symptom-stratified populations of AD patients. Should this trend toward IL-22 elevation be fully substantiated in AD, therapeutic stratification and precision medicine approaches may be conceivable based on serum IL-22 protein concentrations, supporting other investigators' earlier work examining IL-22 mRNA(9). The baseline readability of endogenous IL-22 in HD sera using this ELISA is also highly advantageous.
Ketwords
Atopic dermatitis, IL-22, ELISA, autoimmune, basal level
References
1. Dumoutier L., Van Roost E., Michaux L., Renald J.C., (2000) IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun., 1:488-94, DOI: 101038/sj.gene.6363716
2. Dudakov J.A., Hanash A.M., van den Brink, M.R.M., (2015) Interleukin-22: Immunobiology and Pathology. Ann. Rev. Immunol., 33:747-785, DOI: 10.1146/annurevimmunol-032414-112123
3. Arshad T., Mansur F., Palek R., Manzoor S and Liska V., (2020), A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol., 11:2148, DOI: 10.3389/fimmu.2020.02148
4. Eyerich K., Dimartino V., Cavani A., (2017), IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol., 47(4):607-614, DOI: 10.1002/eji.201646723
5. Li L.J., Gong C., Zhao M.H. Feng B.S. (2014), Role of interleukin-22 in inflammatory bowel disease. World J. Gastroenterol., 20(48):18177-18188, DOI: 10.3748/wjg.v20.i48.18177
6. Brand S., Beigel F., Olszak T., Zitzmann K., Eichhorst S.T., Otte J.M., Diepolder H., Marquardt A., Jagla W., Popp A., Leclair S., Herrmann K., Seiderer J., Ochsenkuhn T., Goke B., Auernhammer C.J., Dambacher J., (2006), IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration, Am. J. Physiol. Gastrointest Liver Physiol., 290:G827-838, DOI: 10.1152/ajpgi.00513.2005
7. Aujla S.J., Chan Y.R., Zheng M., Fei M., Askev D.J., Pociask D.A., Reinhart T.A., McAllister F., Edeal J., Gaus K., Husain S., Kreindler J.L., Dubin P.J., Pilewski J.M., Myerburg M.M., Mason C.A., Iwakura Y., Kolls J.K., (2008), IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia, Nat. Med., 14:275-281, DOI: 10.1038/nm1710
8. Eyerich S., Traidl-Hoffmann C., Behrendt H., Cavani A., Schmidt-Weber C.B., Ring J., Eyerich K., (2017), Novel key cytokines in allergy: IL-17, IL-22. Allergologie Select., 1(1):71-76
9. Brunner P.M., Pavel A.B., Khattri S., Leonard A., Malik K., Rose S., Jim On S., Vekaria A.S., Traidl-Hoffmann C., Singer G.K., Baum D., Gilleaudeau P., Sullivan-Whalen M., Fuentes-Duculan J., Li X., Zheng X., Estrada Y., Garcet S., Wen H.C., Gonzalez J., Coats I., Cueto I., Neumann A.U., Lebwohl M.G., Krueger J.G., Guttman-Yassky E., (2019), Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab
10. Miyagawa I., Nakayamada S., Ueno M., et al., (2022), Impact of serum interleukin-22 as a biomarker for the differential use of molecular targeted drugs in psoriatic arthritis: a retrospective study., Arthritis Res. Ther., 24:86, DOI: 10.1186/s13075-022-02771-4
11. Lavoie T.N., Stewart C.M., Berg K.M., Li Y., Nguyen C.Q., (2011), Expression of interleukin-22 in Sjorgen's syndrome: significant correlation with disease parameters., Scan. J. Immunol., 74(4):377-381, DOI: 10.1111/j.1365-3085.2011.02583.x
12. M Martin J.C., Beriou G., Heslan M., Chauvin C., Utriainen L., Aumeunier A., Scott C.L., Mowat A., Cerovic V., Houston S.A., Leboeuf M., (2014), Interleukin-22 binding protein cells and is strongly induced by retinoic acid., Mucosal Immunol. 7(1):101-113, DOI: 10.1038/mi.2013.28