Diseases Characterized by Interferon Overproduction
PBL’s VeriKine High Sensitivity Human IFN-Beta Serum ELISA (Catalog No. 41415) accurately quantifies low pg/ml levels of human IFN-Beta in complex biological samples including autoimmune sera, normal sera/plasma and tissue culture media (TCM). This ELISA has an assay range of 1.2-150 pg/ml and a lower limit of quantitation (LLOQ) of 1.2 pg/ml. IFN-Beta binds to plates coated with capture antibody and is detected by a biotinylated secondary antibody followed by streptavidin conjugated to horseradish peroxidase (HRP). Tetramethylbenzidine (TMB) serves as the substrate.
Various studies were conducted to assess the robustness, sensitivity and accuracy of this kit.
IFN-Beta concentrations in normal human sera (NHS), Multiple Sclerosis (MS) patient sera and human plasma were measured using Protocol A for improved performance in serum samples. NHS, MS patient sera and plasma in different anticoagulants (disodium EDTA, sodium heparin, sodium citrate) were obtained from Bioreclamation (BioIVT) and the Clinical Research Center of Cape Cod. Mammalian human IFN-Beta standard was provided within the kit. All standards and spikes consist of mammalian cell-expressed IFN-Beta1a.
Results
Figure 1. Typical Standard Curves in Various Matrices. Human IFN-Beta ELISA standard curves were prepared in normal human serum (NHS), tissue culture media (TCM) and standard diluent (SD). The above graph shows the mean of 8 runs in standard diluent and NHS over multiple plates, as well as the mean of 4 runs in TCM. Error bars indicate the standard deviation of the mean.
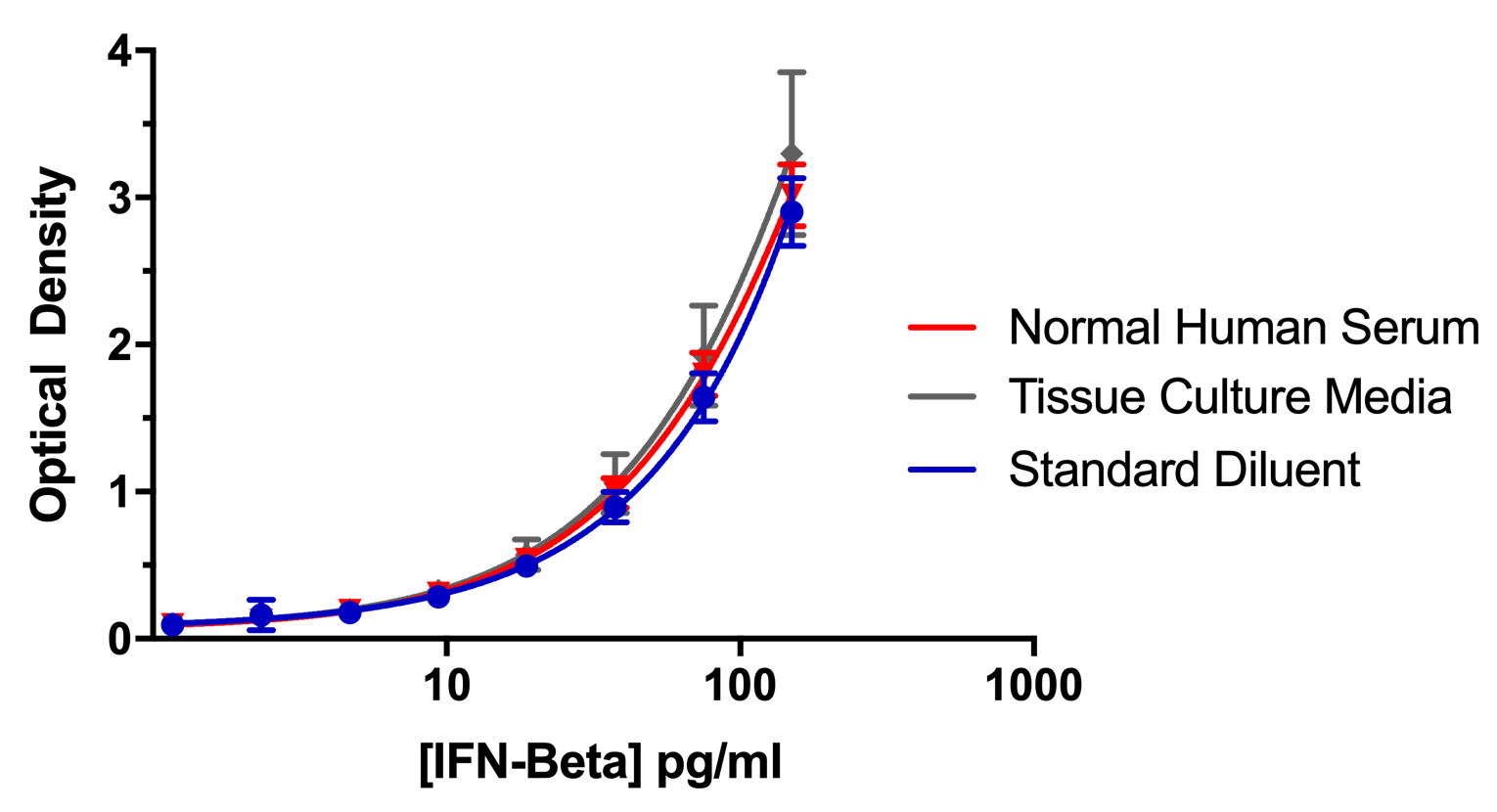
Figure 2. Spike Recovery in Normal Human Serum (NHS). The figure above depicts the mean of 8 runs on multiple plates using one low background lot of NHS. Three target IFN-Beta spike levels were tested: high (100 pg/ml), medium (20 pg/ml) and low (4 pg/ml). Spike concentration was calculated from the standard curve prepared in standard diluent. IFN-Beta was measureable with an accuracy of +/- 20% of the expected value. Error bars indicate the standard error of the mean.

Figure 5. Lot-to-Lot Consistency. Standard curves from 8 different lots of 41415 Human IFN-Beta Standard were evaluated and compared to a recent lot of 41415 (L- 6966). Expired kit lots (denoted by italics and an asterisk (*)) were tested as part of an ongoing stability study. L-6790 and L-6672 were tested within a year of expiration, L- 6428 and L-6530 at 1 year post-expiration, and L-6277 at 2 years post-expiration. IFN- Beta was detectable with an accuracy of +/- 20% of the mean calculated concentration. Error bars indicate the standard deviation between two runs. All concentrations were run twice with the exception of 150 and 1.17 pg/ml.
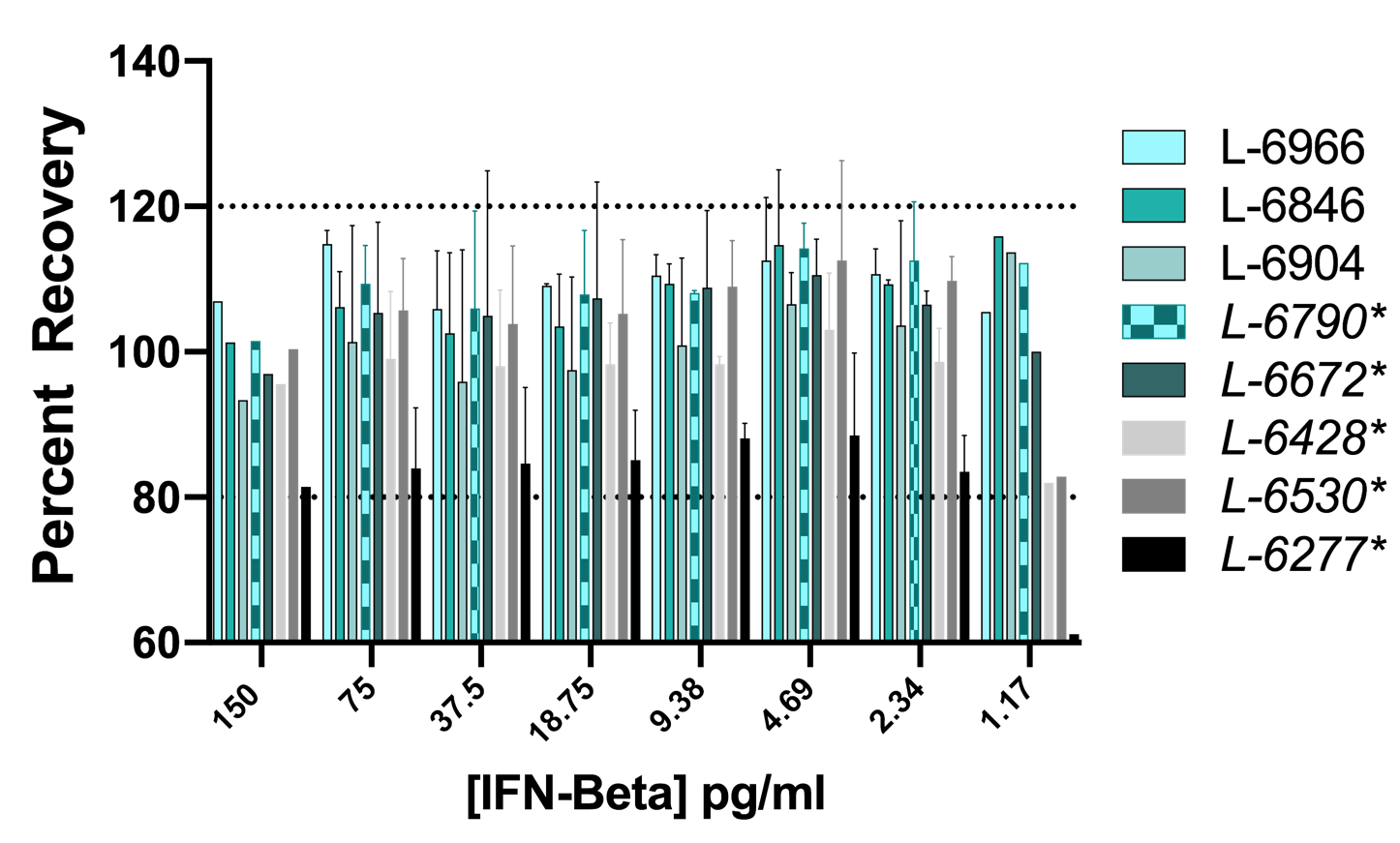
Figure 6. Linearity of Dilution in Normal Human Serum (NHS). 5 independent spikes of 80 pg/ml were prepared using one low background human serum lot. Five two-fold serial dilutions of the spike were performed in standard diluent. Spikes were tested over 3 different days. The concentrations of the variably diluted spikes were calculated from the standard curve in standard diluent. IFN-Beta was detectable with an accuracy of +/- 20% of the expected value with the exception of a single dilution from one run.
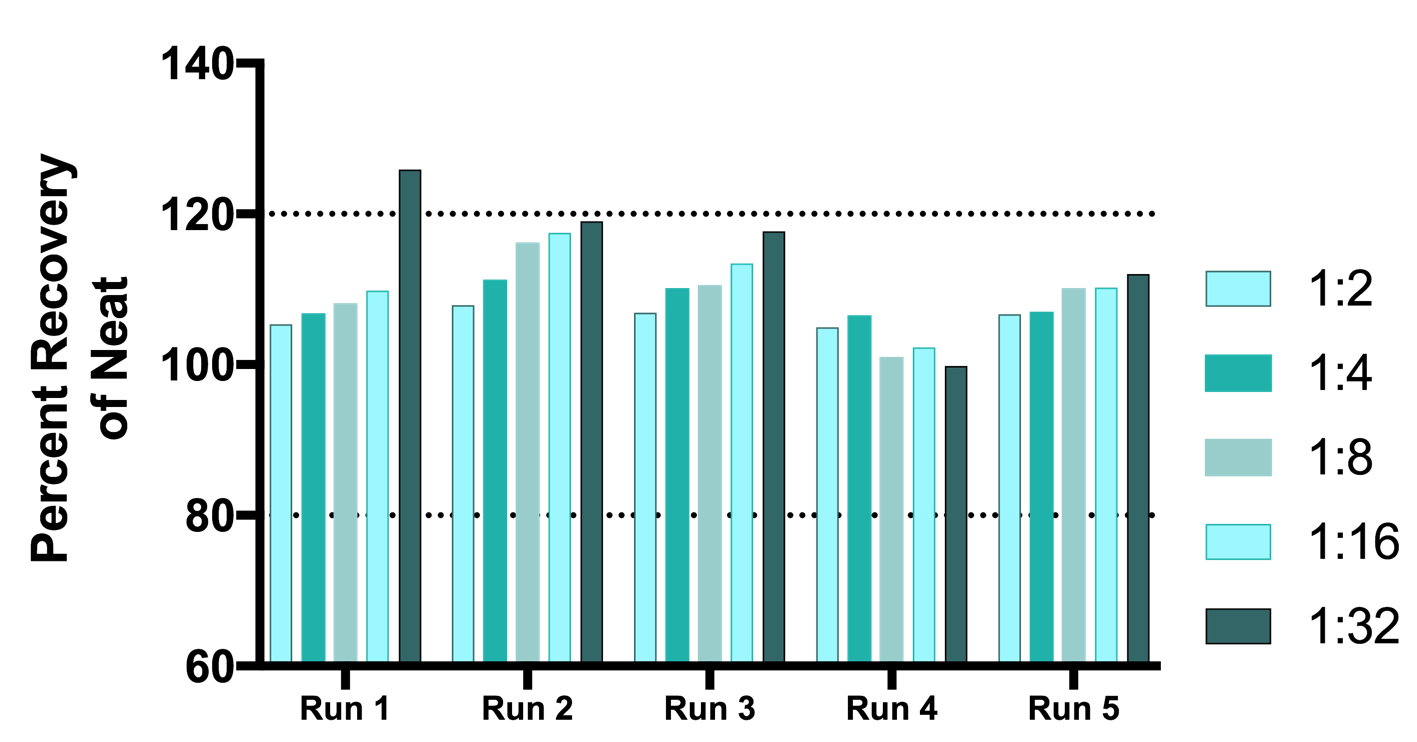
Figure 7. Linearity of Dilution in Multiple Sclerosis (MS) Samples. Assay linearity was tested in 4 different samples of IFN-Beta-positive sera from individual MS patients undergoing IFN-Beta therapy. All samples had been previously tested for IFN-Beta. All samples were run in a single experiment with the exception of Sample 6276 (±) which was run twice. Five or six two-fold serial dilutions were made of each serum sample in standard diluent. The concentrations of IFN-Beta were calculated from the standard curve in standard diluent. IFN-Beta was detectable with an accuracy of +/- 20% of the nominal value. Nominal concentration of dilutions were based on the value measured in the neat sample.
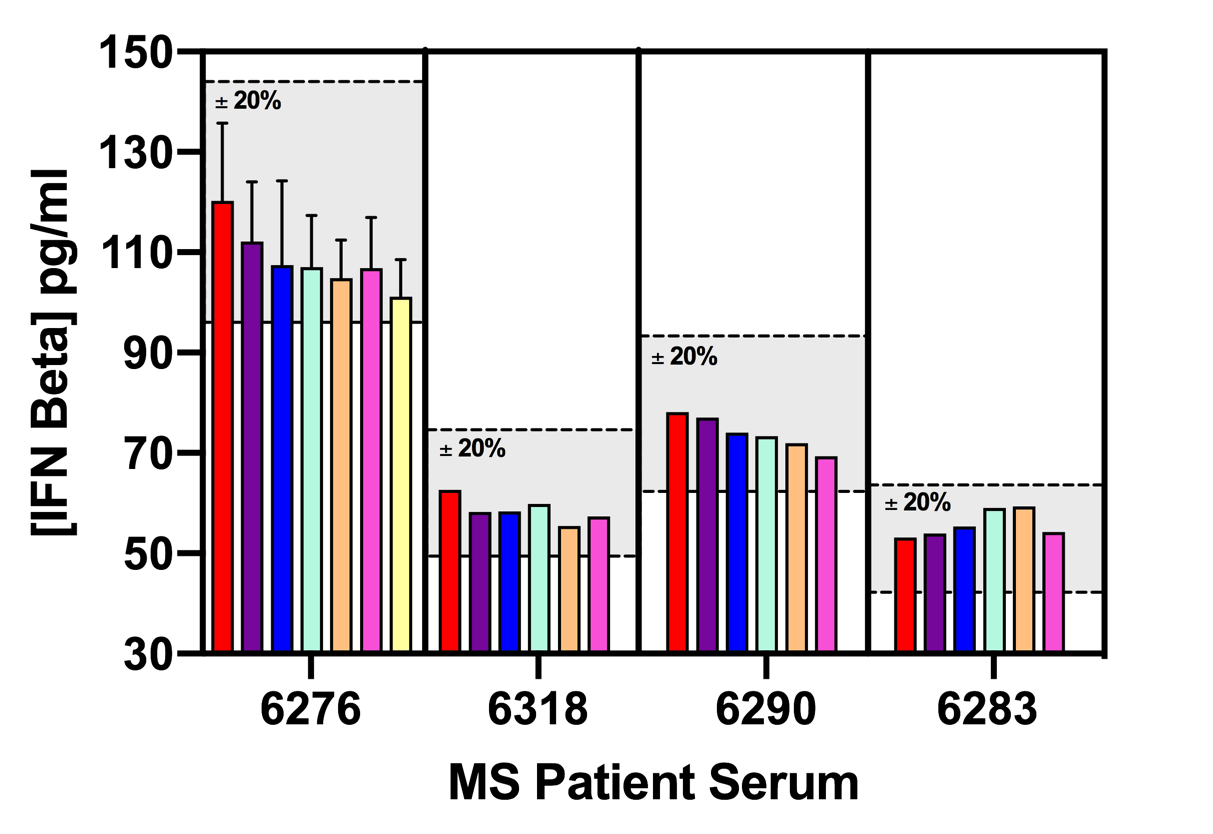
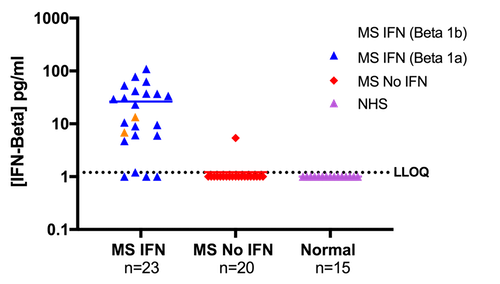
Figure 8. Levels of IFN-Beta Quantified in Normal Human Sera (NHS) and Multiple Sclerosis (MS) Patient Sera. 43 MS serum samples and 15 NHS samples were tested for IFN-Beta. 23 MS patients were on IFN-Beta treatment and 20 were on other therapies. Of the 23 MS patients on IFN-Beta treatment, 2 were on Betaseron (Beta 1b), while the remaining 21 were on Rebif and Avonex (Beta 1a). IFN-Beta was quantifiable in 87% of MS patients on IFN-Beta therapy and 5% of patients on other therapies. All samples found to be below LLOQ are plotted on the graph above at the level of 1.0 pg/ml.
Summary
PBL’s VeriKine High Sensitivity Human Interferon Beta Serum ELISA (Cat. No. 41415) performs well in autoimmune sera, normal serum, tissue culture media (DMEM supplemented with 10% FBS), and in plasma containing the anticoagulants disodium EDTA, sodium heparin or sodium citrate. The ELISA accurately quantifies to ~1.2 pg/ml of IFN- Beta 1a in autoimmune serum. Samples of each of these matrices may be evaluated for unknown concentrations of IFN-Beta within the assay range in bioanalysis studies.
Repeated testing of older and recent kit lots reveal the ELISA’s robustness and sensitivity, as well as lot-to-lot consistency.
Soluble IFNAR2 (sIFNAR2) did not affect the detection of IFN-Beta. The 41415 ELISA appears to measure sIFNAR2-bound IFN-Beta, as well as free IFN-Beta present in serum.
An ongoing stability study demonstrates that the human IFN-Beta standard is stable over time and can be used to accurately measure IFN-Beta over multiple lots.
The Human IFN-Beta ELISA exhibits good Linearity-of-dilution performance both in normal human serum (NHS) and in autoimmune sera, such as that from MS patients. The assay offers precise results over multiple levels of dilutions in complex matrices.
References
1. Piehler, J., Thomas, C., Garcia, K. C., & Schreiber, G. (2012). Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunological reviews, 250(1), 317-34.
2. Hunt, M. & Hunt, R. (2018). Interferon. [online] Available at: http://www.microbiologybook.org/mhunt/interferon.htm.
3. Kasper, L. and Reder, A. (2014). Immunomodulatory activity of interferon-beta. Annals of Clinical and Translational Neurology, 1(8), pp.622-631.
Diseases Characterized by Interferon Overproduction
PBL’s VeriKine High Sensitivity Human IFN-Beta Serum ELISA (Catalog No. 41415) accurately quantifies low pg/ml levels of human IFN-Beta in complex biological samples including autoimmune sera, normal sera/plasma and tissue culture media (TCM). This ELISA has an assay range of 1.2-150 pg/ml and a lower limit of quantitation (LLOQ) of 1.2 pg/ml. IFN-Beta binds to plates coated with capture antibody and is detected by a biotinylated secondary antibody followed by streptavidin conjugated to horseradish peroxidase (HRP). Tetramethylbenzidine (TMB) serves as the substrate.
Various studies were conducted to assess the robustness, sensitivity and accuracy of this kit.
IFN-Beta concentrations in normal human sera (NHS), Multiple Sclerosis (MS) patient sera and human plasma were measured using Protocol A for improved performance in serum samples. NHS, MS patient sera and plasma in different anticoagulants (disodium EDTA, sodium heparin, sodium citrate) were obtained from Bioreclamation (BioIVT) and the Clinical Research Center of Cape Cod. Mammalian human IFN-Beta standard was provided within the kit. All standards and spikes consist of mammalian cell-expressed IFN-Beta1a.
Results
Figure 1. Typical Standard Curves in Various Matrices. Human IFN-Beta ELISA standard curves were prepared in normal human serum (NHS), tissue culture media (TCM) and standard diluent (SD). The above graph shows the mean of 8 runs in standard diluent and NHS over multiple plates, as well as the mean of 4 runs in TCM. Error bars indicate the standard deviation of the mean.

Figure 2. Spike Recovery in Normal Human Serum (NHS). The figure above depicts the mean of 8 runs on multiple plates using one low background lot of NHS. Three target IFN-Beta spike levels were tested: high (100 pg/ml), medium (20 pg/ml) and low (4 pg/ml). Spike concentration was calculated from the standard curve prepared in standard diluent. IFN-Beta was measureable with an accuracy of +/- 20% of the expected value. Error bars indicate the standard error of the mean.

Figure 5. Lot-to-Lot Consistency. Standard curves from 8 different lots of 41415 Human IFN-Beta Standard were evaluated and compared to a recent lot of 41415 (L- 6966). Expired kit lots (denoted by italics and an asterisk (*)) were tested as part of an ongoing stability study. L-6790 and L-6672 were tested within a year of expiration, L- 6428 and L-6530 at 1 year post-expiration, and L-6277 at 2 years post-expiration. IFN- Beta was detectable with an accuracy of +/- 20% of the mean calculated concentration. Error bars indicate the standard deviation between two runs. All concentrations were run twice with the exception of 150 and 1.17 pg/ml.

Figure 6. Linearity of Dilution in Normal Human Serum (NHS). 5 independent spikes of 80 pg/ml were prepared using one low background human serum lot. Five two-fold serial dilutions of the spike were performed in standard diluent. Spikes were tested over 3 different days. The concentrations of the variably diluted spikes were calculated from the standard curve in standard diluent. IFN-Beta was detectable with an accuracy of +/- 20% of the expected value with the exception of a single dilution from one run.
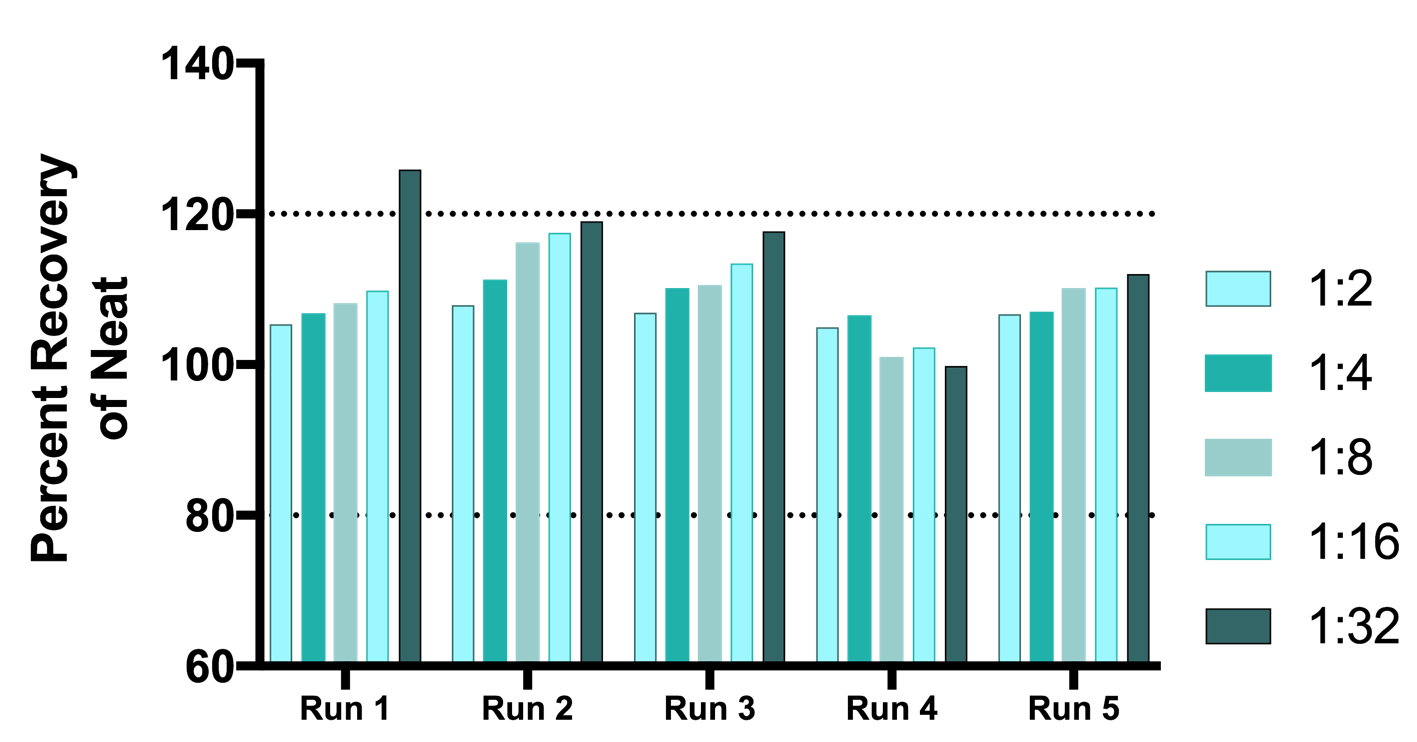
Figure 7. Linearity of Dilution in Multiple Sclerosis (MS) Samples. Assay linearity was tested in 4 different samples of IFN-Beta-positive sera from individual MS patients undergoing IFN-Beta therapy. All samples had been previously tested for IFN-Beta. All samples were run in a single experiment with the exception of Sample 6276 (±) which was run twice. Five or six two-fold serial dilutions were made of each serum sample in standard diluent. The concentrations of IFN-Beta were calculated from the standard curve in standard diluent. IFN-Beta was detectable with an accuracy of +/- 20% of the nominal value. Nominal concentration of dilutions were based on the value measured in the neat sample.

Figure 8. Levels of IFN-Beta Quantified in Normal Human Sera (NHS) and Multiple Sclerosis (MS) Patient Sera. 43 MS serum samples and 15 NHS samples were tested for IFN-Beta. 23 MS patients were on IFN-Beta treatment and 20 were on other therapies. Of the 23 MS patients on IFN-Beta treatment, 2 were on Betaseron (Beta 1b), while the remaining 21 were on Rebif and Avonex (Beta 1a). IFN-Beta was quantifiable in 87% of MS patients on IFN-Beta therapy and 5% of patients on other therapies. All samples found to be below LLOQ are plotted on the graph above at the level of 1.0 pg/ml.

Summary
PBL’s VeriKine High Sensitivity Human Interferon Beta Serum ELISA (Cat. No. 41415) performs well in autoimmune sera, normal serum, tissue culture media (DMEM supplemented with 10% FBS), and in plasma containing the anticoagulants disodium EDTA, sodium heparin or sodium citrate. The ELISA accurately quantifies to ~1.2 pg/ml of IFN- Beta 1a in autoimmune serum. Samples of each of these matrices may be evaluated for unknown concentrations of IFN-Beta within the assay range in bioanalysis studies.
Repeated testing of older and recent kit lots reveal the ELISA’s robustness and sensitivity, as well as lot-to-lot consistency.
Soluble IFNAR2 (sIFNAR2) did not affect the detection of IFN-Beta. The 41415 ELISA appears to measure sIFNAR2-bound IFN-Beta, as well as free IFN-Beta present in serum.
An ongoing stability study demonstrates that the human IFN-Beta standard is stable over time and can be used to accurately measure IFN-Beta over multiple lots.
The Human IFN-Beta ELISA exhibits good Linearity-of-dilution performance both in normal human serum (NHS) and in autoimmune sera, such as that from MS patients. The assay offers precise results over multiple levels of dilutions in complex matrices.
References
1. Piehler, J., Thomas, C., Garcia, K. C., & Schreiber, G. (2012). Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunological reviews, 250(1), 317-34.
2. Hunt, M. & Hunt, R. (2018). Interferon. [online] Available at: http://www.microbiologybook.org/mhunt/interferon.htm.
3. Kasper, L. and Reder, A. (2014). Immunomodulatory activity of interferon-beta. Annals of Clinical and Translational Neurology, 1(8), pp.622-631.

Related Article
Validation Of a Highly Sensitive Immunoassay For The Quantitation Of Interferon Beta In Autoimmune Sera
Read Article
