Human IFN-Beta ELISA Kit, High Sensitivity (Serum, Plasma, TCM)
Catalog Number: 41415
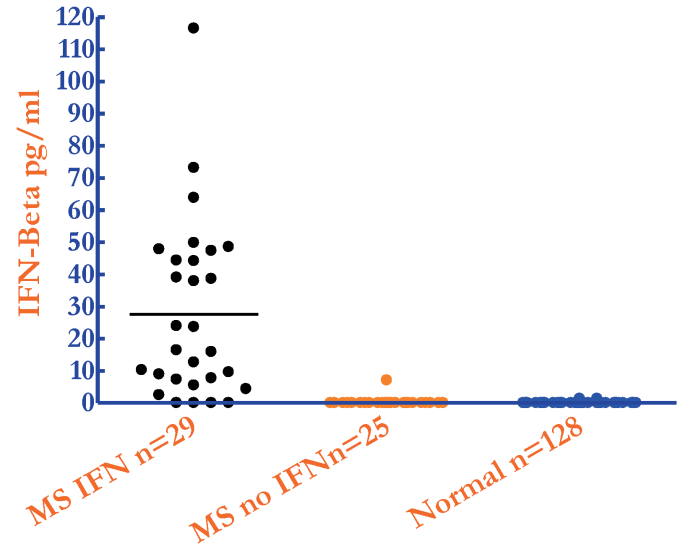
This Human IFN-Beta ELISA measures interferon beta levels in human serum, plasma, and tissue culture samples with 1.2 pg/ml LLOQ sensitivity. This kit has been validated for the measurement of IFN-Beta in autoimmune serum and of trademarked IFN-Beta 1a and IFN-Beta 1b therapeutic molecules in human serum. There is no detection inhibition by 3-log excess of soluble IFNAR2 protein.
Product Name: VeriKine-HS Human Interferon-Beta ELISA Kit (Serum, Plasma, TCM)
$725.00
Product Info
| Matrix Compatibility | Serum, Plasma, Tissue Culture Media (TCM) |
|---|---|
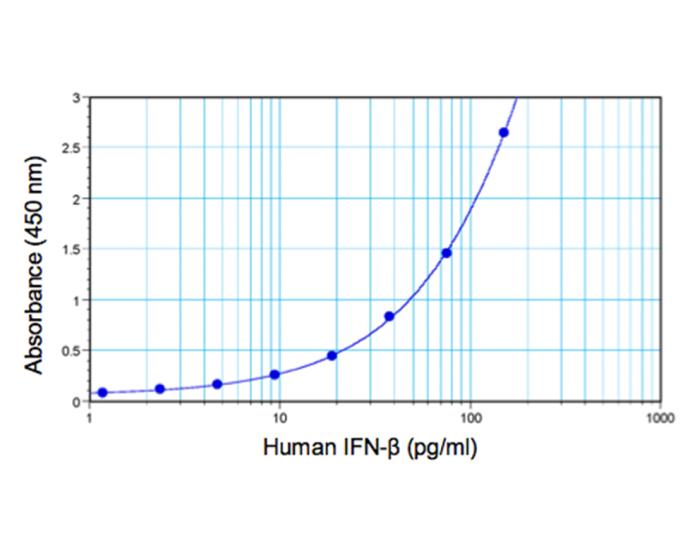
| Assay Range | Protocol A: 1.2 - 150 pg/ml (for improved serum performance) Protocol B: 2.3 - 150 pg/ml |
| LLOQ | 1.2 pg/ml Need more sensitivity? Check out our Sample Testing Services |
| Assay Length | Protocol A: 3 hours, 30 minutes Protocol B: 3 hours |
| Specificity | Human IFN-β |
The VeriKine-HS Human IFN-Beta ELISA Kit is designed to measure low or basal levels of Human IFN-Beta in autoimmune disease sera, healthy serum/plasma, or tissue culture media samples with 1.2 pg/ml LLOQ sensitivity.
This assay is suitable for the measurement of trademarked therapeutic molecules in human serum samples. Researchers and clinical investigators examining a) the pharmacokinetics of IFN-Beta molecules, b) IFN-Beta as a biomarker, or c) IFN-Beta as a pharmacodynamic marker of TLR agent or other immune response modifier activity will find this immunoassay to be an essential laboratory tool.
*For a TCM-compatible ELISA, we recommend our Human IFN-Beta ELISA Kit, High Sensitivity (Cat. No. 41435-1).
Specifications
| CVs and Spike Recovery | Inter-Assay < 8%
Spike Recovery > 90% in Serum |
|---|---|
Cross-reactivity
| No cross-reactivity detected with:
|
| Synonyms | Human Beta Interferon, Human Fibroblast IFN, Human IFN Beta, Human Fibroblast Interferon, Human Beta IFN, Human Type I Interferon Beta, Human IFN B |
| Storage | 2-8°C |
| Expiration Date | 12 months from the date of manufacture |
| Shipping Condition | Wet Ice |
Materials Provided
- Pre-coated microtiter plate
- Plate Sealers
- Wash Solution Concentrate
- Human Interferon Beta 1a Standard, 100,000 pg/ml
- Standard Diluent
- Sample Buffer
- Antibody Concentrate
- HRP Conjugate Concentrate
- Diluent Additive III (for use in Protocol A)
- Assay Diluent
- TMB Substrate
- Stop Solution
Additional Materials Required (Not Provided)
- Microplate reader capable of reading an OD at a wavelength of 450 nm
- Variable volume microtiter pipettes
- Adjustable multichannel pipette (50-300 μl)
- Reagent reservoirs
- Wash bottle or plate washing system
- Distilled or deionized water
- Serological pipettes (1, 5, 10 or 25 ml)
- Disposable pipette tips (polypropylene)
- Plate shaker
Tech Info & Data
Background
Interferon beta (IFN-Beta, IFNb) is part of the first wave of cytokine response in cells. Pathogen infection can result in the activation of interferon regulatory factor 3 (IRF3) which functions in trans to activate IFN-Beta gene transcription. IFN-Beta is biologically unique when compared to other interferons and studies have shown that IFN-Beta has overlapping and distinct gene expression patterns as compared to IFN-Alpha. It appears that IFN-Beta binds to the Type I IFN receptor with higher affinity than the other Type I IFNs and it may also regulate receptor internalization in a different manner. Additionally, IFN-Beta has long been known to inhibit viral replication as part of the body’s innate antiviral response and is used as a therapeutic for the treatment of Multiple Sclerosis (MS) and some tumors.
Citations
Posters
- Performance Characterization Of A High Sensitivity Human Interferon Beta ELISA Kit In Healthy Serum, Patient Serum And Plasma Samples (link)
- Validation of a Highly Sensitive Immunoassay for the Quantitation of Interferon Beta in Autoimmune Sera (link)
- Optimization and Validation of an ELISA kit for the Quantification of Four Interferon-Beta (IFN-β) Marketed Compounds in Human Serum (link)
57 Citations
- Yu, Y. et al., (2024), "Combining VPS34 inhibitors with STING agonists enhances type I interferon signaling and anti-tumor efficacy", Mol Oncol., PMID: 38506049, DOI: 10.1002/1878-0261.13619 (link)
- Lei, X. et al., (2024), "CD4+T cells produce IFN-I to license cDC1s for induction of cytotoxic T-cell activity in human tumors", Cell Mol Immunol., PMID: 38383773, DOI: 10.1038/s41423-024-01133-1 (link)
- Morihiro, K. et al., (2024), "RNA Oncological Therapeutics: Intracellular Hairpin RNA Assembly Enables MicroRNA-Triggered Anticancer Functionality", J Am Chem Soc. PMID: 38170469, DOI: 10.1021/jacs.3c09524 (link)
- Rohilla, A. et al., (2023), "Structure-based virtual screening and in vitro validation of inhibitors of cyclic dinucleotide phosphodiesterases ENPP1 and CdnP", Micro Spectr., e0201223, PMID: 38095464, DOI: 10.1128/spectrum.02012-23 (link)
- Smith, KER., et al., (2023), "A phase I oncolytic virus trial with vesicular stomatitis virus expressing human interferon beta and tyrosinase related protein 1 administered intratumorally and intravenously in uveal melanomia: safety, efficacy, and T cell responses", Front Immunol., 14:1279387, DOI: 10.3389/fimmun.2023.1279387 (link)
- Grunhagel, B., et al., (2023), "Reduction of IFN-I Responses by Plasmacytoid Dendritic Cells in a Longitudinal Trans Men Cohort, iScience, DOI: 10.1016/j.isci.2023.108209 (link)
- Nilsen, K.E., et al., (2023), "Peptide derived from SLAMF1 prevents TLR4-mediated inflammation in vitro and in vivo", Life Sci Alliance, 6(12):e202302164, PMID: 37788908, DOI: 10.26508/isa.202302164 (link)
- Ullah, T.R. et al., (2023), "Pharmacological inhibition of TBK1/IKKε blunts immunopathology in a murine model of SARS-CoV-2 infection", Nat Commun. 14(1):5666, PMID: 37723181, DOI: 10.1038/s41467-023-41381-9 (link)
- Arbore, G. et al., (2023), "Pre-existing immunity drives the response to neoadjuvant chemotherapy in esophageal adenocarcinoma", Cancer Res., CAN-23-0356, PMID: 37350667, DOI: 10.1158/0008-5472.CAN-23-0356 (link)
- Rajamanickam, A. et al., (2022), "Restoration of dendritic cell homeostasis and Type I/Type III interferon levels in convalescent COVID-19 individuals, BMC Immunol., 23(1):51, PMID: 36289478, DOI: 10.1186/s12865-022-00526-z (link)
- Nagaoka, N., et al., (2022), "Effect of Casirivimab/Imdevimab Treatment on Serum Type I Interferon Levels in SARS-CoV-2 Infection", Viruses, 14(7):1399, DOI: 10.3390/v14071399 (link)
- Dickey, L. et al., (2022), HIV-1-induced type I IFNs promote viral latency in macrophages, J. Leukoc. Biol. PMID:35588262, DOI:10.1002/JLB.4MA0422-616R (link)
- Steiner, A. et al., (2022), Deficiency in coatomer complex I causes aberrant activation of STING signalling, Nature Communications, 13:2321, PMID: 35484149, DOI: 10.1038/s41467-022-29946-6 (link)
- Akoi, Y. et al., (2022), Evaluation of the Relationships between Intestinal Regional Lymph Nodes and Immune Responses in Viral Infections in Children, Int. J. Mol. Sci., 23:318, DOI: 10.3390/ijms23010318 (link)
- Jablonska, A., et al., (2021), The TLR9 2848C/T Polymorphism Is Associated with the CMV DNAemia among HIV/CMV Co-Infected Patients, Cells, 10:2360, DOI: 10.3390/cells10092360, (link)
- Dorgham, et al. (2021). Considering Personalized Interferon Beta Therapy for COVID-19. Antimicrobial Agents and Chemotherapy, 3 pgs. PMID: 21321205. (link)
- Contoli, M. et al., (2021), Blood Interferon-a Levels and Severity, Outcomes, and Inflammatory Profiles in Hospitalized COVID-19 Patients, Front. Immunol., PMID: 33767713, DOI: 10.3389/fimmu.2021.648004 (link)
- Terajima, H et al., (2021), N6-methyladenosine promotes induction of ADAR1-mediated A-to-I RNA editing to supress aberrant antiviral innate immune response, PLoS Biol., 19(7):e3001292, PMID:34324489 (link)
- Blanco-Melo, D. et al., (2020), Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19, Cell, 181(5):1036, PMID: 32416070, DOI: 10.1016/j.cell.2020.04.026 (link)
- Dubey, et al. (2020). Specific protein-protein interactions limit the cutaneous iontophoretic transport of interferon beta-1B and a poly-ARG interferon beta-1B analogue. International Journal of Pharmaceutics, 6 pgs. PMID: no PMID. (link)
- Newling, et al. (2019). Dysregulated Fcγ receptor IIa-induced cytokine production in dendritic cells of lupus nephritis patients. Clinical and Experimental Immunology, 11 pgs. PMID: 31509231. link)
- Skjeflo, et al. (2019). Phagocytosis of live and dead Escherichia coli and Staphylococcus aureus in human whole blood is markedly reduced by combined inhibition of C5aR1 and CD14. Molecular Immunology, 9 pgs. PMID: 31102985. (link)
- Dubois, et al. (2019). The Nonstructural NS1 Protein of Influenza Viruses Modulates TP53 Splicing through Host Factor CPSF4. JVI, 35 pgs. PMID: 30651364. (link)
- Gannon, et al. (2018). Identification of ADAR1 adenosine deaminase dependency in a subset of cancer cells. Nature Communications, 10 pgs. PMID: 30575730. (link)
- Colavita, Francesca, et al. (2018). Overproduction of IL-6 and Type-I IFN in a Lethal Case of Chikungunya Virus Infection in an Elderly Man During the 2017 Italian Outbreak. Open Forum Infectious Diseases, 21 pgs. PMID: 30539034. (link)
- Merindol, Natacha, et al. (2018). HIV-1 Capsids from B27/B57+ Elite Controllers Escape Mx2 but are Targeted by TRIM5-alpha, Leading to the Induction of an Antiviral State. PLOS Pathogens, 22 pgs. PMID: 30419009. (link)
- Zhang, Guoliang, et al. (2018). A proline deletion in IFNAR1 impairs IFN-signaling and underlies increased resistance to tuberculosis in humans. Nature Communications, 9 pgs. PMID: 29311663. (link)
- Ager, Casey (2018). Intratumoral STING Activation Sensitizes Poorly Immunogenic Cancers to Checkpoint Blockade Immunotherapy. University of Texas, 229 pgs. PMID: no PMID. (link)
- Ehrnstroem, Birgitta, et al. (2017). Toll-Like Receptor 8 Is a Major Sensor of Group B Streptococcus But Not Escherichia coli in Human Primary Monocytes and Macrophages. Frontiers in Immunology, 14 pgs. PMID: 29042860. (link)
- Kondoh, Tatsunari, et al. (2017). Putative endogenous filovirus VP35-like protein potentially functions as an IFN antagonist but not a polymerase cofactor. PLOS One, 17 pgs. PMID: 29040311. (link)
- Brown, Michael, et al. (2017). Cancer Immunotherapy with Recombinant Poliovirus Induces IFN-Dominant Activation of Dendritic Cells and Tumor Antigen-Specific CTLs. Sci Transl Med, 31 pgs. PMID: 28931654. (link)
- Cheng, Liang, et al. (2017). Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction during chronic HIV-1 infection. JCI Insight, 13 pgs. PMID: 28614789. (link)
- Luan, Liming, et al. (2017). Comparative Transcriptome Profiles of Human Blood in Response to the Toll-like Receptor 4 Ligands Lipopolysaccharide and Monophosphoryl Lipid A. Scientific Reports, 16 pgs. PMID: 28053314. (link)
- Gusella, Luca, et al. (2016). Prothymosin-α Variants Elicit Anti-HIV-1 Response via TLR4 Dependent and Independent Pathways. PLOS One, 17 pgs. PMID: 27310139. (link)
- GuhaSarkar, Dwijit, et al. (2016). Systemic AAV9-IFNβ gene delivery treats highly invasive glioblastoma. Neuro-Oncology, 11 pgs. PMID: 27194146. (link)
- Vanheule, Vincent, et al. (2016). Basic chemokine-derived glycosaminoglycan binding peptides exert antiviral properties against dengue virus serotype 2, herpes simplex virus-1 and respiratory syncytial virus. Biochemical Pharmacology, pgs. PMID: 26551597. (link)
- Chiappinelli, Katherine, et al. (2015). Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. CellPress, 23 pgs. PMID: 26317466. (link)
- Dauletbaev, Nurlan, et al. (2015). Stimulation of the RIG-I/MAVS Pathway by Polyinosinic:Polycytidylic Acid Upregulates IFN-β in Airway Epithelial Cells with Minimal Costimulation of IL-8. Journal of Immunology, 14 pgs. PMID: 26283481. (link)
- Spengler, Jessica, et al. (2015). RIG-I Mediates an Antiviral Response to Crimean-Congo Hemorrhagic Fever Virus. JVI, 11 pgs. PMID: 26223644. (link)
- Eigenbrod, Tatjana, et al. (2015). TLR8 Senses Bacterial RNA in Human Monocytes and Plays a Nonredundant Role for Recognition of Streptococcus pyogenes. Journal of Immunology, 9 pgs. PMID: 26101323. (link)
- Bergstrom, Bjarte, et al. (2015). TLR8 Senses Staphylococcus aureus RNA in Human Primary Monocytes and Macrophages and Induces IFN-β Production via a TAK1-IKKβ-IRF5 Signaling Pathway. Journal of Immunology, 13 pgs. PMID: 26085680. (link)
- Olaisen, Camilla, et al. (2015). PCNA-interacting peptides reduce Akt phosphorylation and TLR-mediated cytokine secretion suggesting a role of PCNA in cellular signaling. Cellular Signaling, 10 pgs. PMID: 25797046. (link)
- White, Michael, et al. (2014). Apoptotic Caspases Suppress mtDNA-Induced STING-Mediated Type I IFN Production. CellPress, 27 pgs. PMID: 25525874. (link)
- Wolferstaetter, Michael, et al. (2014). Recombinant Modified Vaccinia Virus Ankara Generating Excess Early Double-Stranded RNA Transiently Activates Protein Kinase R and Triggers Enhanced Innate Immune Responses. JVI, 16 pgs. PMID: 25297997. (link)
- Asgari, et al. (2013). Clinical Study: Interferon Alpha Association with Neuromyelitis Optica. Clinical and Developmental Immunology, 7 pgs. PMID: 24348680. (link)
- Nakayama, Masanori, et al. (2013). IL-32-PAR2 axis is an innate immunity sensor providing alternative signaling for LPS-TRIF axis. Scientific Reports, 10 pgs. PMID: 24129891. (link)
- Lee, et al. (2013). xSite-Specific PEGylation Enhances the Pharmacokinetic Properties and Antitumor Activity of Interferon Beta-1b. Journal of Interferon & Cytokine Research, pgs. PMID: 23962003. (link)
- Kuri, et al. (2013). Influenza A Virus-Mediated Priming Enhances Cytokine Secretion by Human Dendritic Cells Infected with Streptococcus pneumoniae. Cellular Microbiology, 16 pgs. PMID: 23421931. (link)
- Weix, et al. (2013). The Physiologic Increase in Expression of Some Type I IFN-Inducible Genes During Pregnancy is Not Associated with Improved Disease Activity in Pregnant Patients with Rheumatoid Arthritis. Translational Research. PMID: no PMID. (link)
- Wang, et al. (2012). Application of Secretary Luciferase Labeled Orthotopic Transplant Model of Hepatocellular Carcinoma to Evaluate Tumor Response to Interferon-beta Gene Therapy. Sheng Wu Gong Cheng Xue Bao. PMID: 23311138. (link)
- Tian, Ren-Rong, et al. (2012). IFN-lambda Inhibits HIV-1 Integration and Post-Transcriptional Events In Vitro, but there is Only Limited In Vivo Repression of Viral Production. Antiviral Research. PMID: 22584351. (link)
- Chiappinelli, et al. (2012). Reduced DICER1 Elicits an Interferon Response in Endometrial Cancer Cells. Molecular Cancer Research, 11 pgs. PMID: 22252463. (link)
- Tarassishin, et al. (2012). Interferon Regulatory Factor 3 Inhibits Astrocyte Inflammatory Gene Expression Through Suppression of the Proinflammatory miR-155 and miR-155*. GLIA. PMID: 22170100. (link)
- Sherwood, et al. (2012). Interferon Treatment Suppresses Enteric Adenovirus Infection in a Model Gastrointestinal Cell-Culture System. Journal of General Virology. PMID: 22158877. (link)
- Tarassishin, et al. (2011). Interferon Regulatory Factor 3 Plays an Anti-Inflammatory Role in Microglia by Activating the PI3K/Akt Pathway. Journal of Neuroinflammation. PMID: 22208359. (link)
- Bauler, et al. (2011). IFN-beta Mediates Suppression of IL-12p40 in Human Dendritic Cells Following Infection with Virulent Francisella tularensis. Journal of Immunology, 12 pgs. PMID: 21753150. (link)
- Muto, et al. (2011). Human Papillomavirus Type 16 E5 Protein Induces Expression of Beta Interferon Through Interferon Regulatory Factor 1 in Human Keratinocytes. JVI, 11 pgs. PMID: 21389130. (link)
- Cervantes, et al. (2011). Phagosomal Signaling by Borrelia burgdorferi in Human Monocytes Involves Toll-like Receptor (TLR) 2 and TLR8 Cooperativity and TLR8-Mediated Induction of IFN-beta. PNAS, 6 pgs. PMID: 21321205. (link)
Background Literature:
- Krause CD, Pestka S. Evolution of the Class 2 cytokines and receptors, and discovery of new friends and relatives. Pharmacol Ther. 2005 Jun;106(3):299-346.
- Weinstock-Guttman B, Ramanathan M, Zivadinov R. Interferon-beta treatment for relapsing multiple sclerosis. Expert Opin Biol Ther. 2008 Sep;8(9):1435-47.
- Lee MS, Kim YJ. Pattern-recognition receptor signaling initiated from extracellular, membrane, and cytoplasmic space. Mol Cells. 2007 Feb 28;23(1):1-10.
- Heim MH. The Jak-STAT pathway: cytokine signaling from the receptor to the nucleus. J Recept Signal Transduct Res. 1999 Jan-Jul;19(1-4):75-120.
- Taniguchi T, Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. 2002 Feb;14(1):111-6.
- Lewerenz M, Mogensen KE, Uzé G. Shared receptor components but distinct complexes for alpha and beta interferons. J Mol Biol. 1998 Sep 25;282(3):585-99.
- Jaks E, Gavutis M, Uzé G, Martal J, Piehler J. Differential receptor subunit affinities of type I interferons govern differential signal activation. J Mol Biol. 2007 Feb 16;366 (2):525- 39.
- Marijanovic Z, Ragimbeau J, van der Heyden J, Uzé G, Pellegrini S. Comparable potency of IFNalpha2 and IFN beta on immediate JAK/STAT activation but differential downregulation of IFNAR2. Biochem J. 2007 Oct 1;407(1):141-51.
- Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001 May; 2(5):378-86.
- Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey JL. Variables That Affect Assays for Plasma Cytokines and Soluble Activation Markers. Clin Diagn Lab Immunol. 1999 Jan: (6)1:89-95.