Cynomolgus/Rhesus IFN-Alpha ELISA Kit (Serum, Plasma, TCM)
Catalog Number: 46100
This ELISA quantifies Cynomolgus and Rhesus IFN-Alpha 2 in serum, plasma, and tissue culture media (TCM). Recombinant Cynomolgus/Rhesus IFN-Alpha 2 is provided as the ELISA standard.
Product Name: VeriKine Cynomolgus/Rhesus Interferon-Alpha ELISA Kit (Serum, Plasma, TCM)
$795.00
Product Info
| Matrix Compatibility | Serum, Plasma, Cell Culture Supernatant |
|---|---|
| Assay Range | 25 - 1600 pg/ml |
| Assay Length | 3 hours, 15 minutes |
| Specificity | Cynomolgus (Macaca fascicularis) and Rhesus (Macaca mulatta) IFN-Alpha 2 |
| CVs |
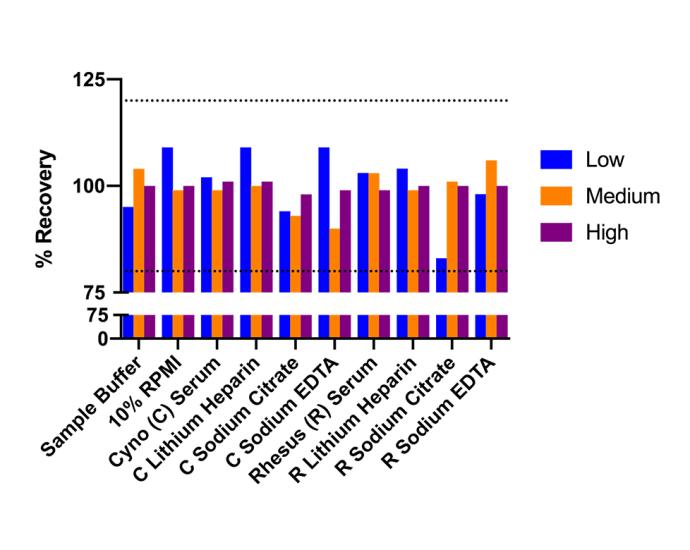
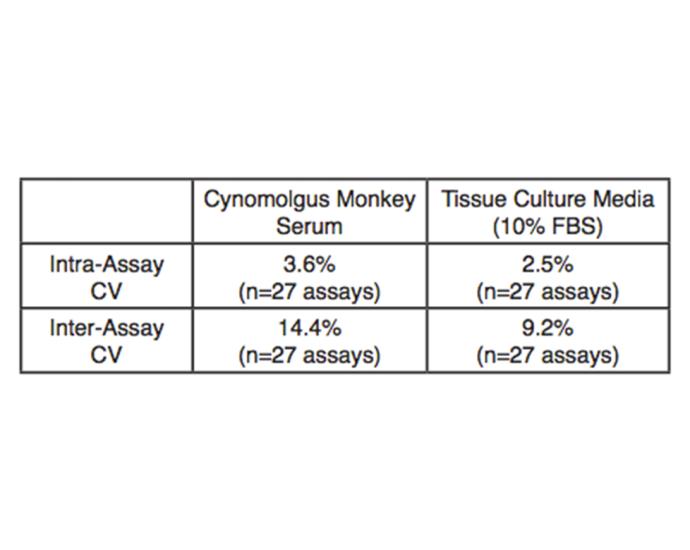
Inter-Assay < 9.2% (TCM); 14.4% (serum)
Average Spike Recovery: 102% in Serum |
The VeriKine Cynomolgus/Rhesus IFN-Alpha Serum ELISA has been developed to analyze the presence of non-human primate IFN-Alpha in tissue culture media, serum, or plasma samples by sandwich enzyme-linked immunosorbent assay (ELISA). The kit is based on an ELISA with a biotinylated anti-detection antibody and a streptavidin horseradish peroxidase (HRP). Tetramethylbenzidine (TMB) is the substrate. The assay is based on PBL’s Rhesus/Cynomolgus IFNA-α2 (PBL 14110), which has been calibrated in reference to the International Standard to Human Interferon Alpha-2a.
As such, it should prove an important tool in virology, immunomodulation and immunotoxicology studies conducted in non-human primates.
Note: The lowest limit of quantitation (LLOQ) is 25 pg/ml. Concentrations of unknown samples that measure < 25 pg/ml are suspect.
Specifications
| CVs and Spike Recovery |
Inter-Assay < 9.2% (TCM); 14.4% (serum)
Average Spike Recovery 102% in Serum |
|---|---|
| Cross-reactivity |
Strong cross-reactivity with
No cross-reactivity against
|
| Storage | 2-8°C |
| Expiration Date | 9 months from the date of manufacture |
| Shipping Condition | Wet Ice |
Materials Provided
- Pre-coated microtiter plate
- Plate Sealers
- Wash Solution Concentrate
- Rhesus/Cyno IFN-Alpha 2 Standard, 10,000 pg/ml
- Standard Diluent
- Sample Buffer
- Antibody Concentrate
- HRP Conjugate Concentrate
- Concentrate Diluent
- TMB Substrate
- Stop Solution
Additional Materials Required (Not Provided)
- Microplate reader capable of reading an OD at a wavelength of 450 nm
- Variable volume microtiter pipettes
- Adjustable multichannel pipette (50-300 μl)
- Reagent reservoirs
- Wash bottle or plate washing system
- Distilled or deionized water
- Serological pipettes (1, 5, 10 or 25 ml)
- Disposable pipette tips (polypropylene)
- Plate shaker
Tech Info & Data
Application Note
White Paper (by request)
- Utility and Application of the VeriKine Cynomolgus/Rhesus Interferon Alpha Serum ELISA
Background
Interferons (IFNs) are a group of cytokines which exhibit pleiotropic activities that play major roles in both innate and adaptive immunity. Type I IFNs consist of at least one IFN-β gene and protein as well as multiple IFN-α genes and proteins in most vertebrate species1.
IFN-α expression and secretion are primarily induced by signaling events processed through pattern recognition receptors such as the Toll-like and RIG-I like receptors (TLR and RLR, respectively). While IFN-α can be produced by most cell types, strong evidence suggests that plasmacytoid dendritic cells are a major source of IFN-α in vivo2.
The interferon alpha gene family is comprised of multiple distinct genes that occupy a single chromosomal locus. All sequenced vertebrate species possess a large number of distinct alpha genes suggesting a biological significance to maintaining multiple copies of IFN-Alpha genes. Published reports have shown the expression patterns of the individual IFN-Alpha proteins and their cell-specific function can vary suggesting unique properties of each gene in a cell-dependent manner.
Following expression and secretion, IFN-α binds to a heterodimeric receptor chain consisting of IFNAR1 and IFNAR2 subunits on proximal and distal cell surfaces. Receptor binding promotes a signal transduction cascade consisting of components of the JAK-STAT signaling pathway. Hundreds of genes are regulated subsequent to binding of the IFNAR receptor subunits to IFN-α, thus leading to the antiviral, anti-proliferative and immunomodulatory activities of the cytokine.
Two nonhuman primate species, Rhesus (Macaca mulatta) and Cynomolgus (Macaca fascicularis) macaques, are sufficiently genetically similar to humans that they are emerging as highly relevant animal models for studying various aspects of human physiology3. For example, macaques provide valuable surrogate models for examining the pathogenicity of human viral infections such as the 1918 pandemic influenza virus4. Furthermore, macaques are included in the evaluation of many new therapeutic agents aimed at modulating host immunity to either enhance or dampen immune responses5,6.
These primates also serve as important immunotoxicological models in the testing of human pharmaceuticals7.
Citations
15 Citations:
- King, H. et al., (2023), "Immune Activation Profiles Elicited by Distinct, Repeated TLR Agnoist Infusions in Rhesus Macaques", J. Immunol., ji:2300424, PMID: 37861342, DOI: 10.4049/jimmunol.2300424 (link)
- Fletcher, P. et al., (2023), Single-dose VSV-based vaccine protects cynomolgus macaques from disease after Tai Forest virus infection, Emerg Microbes Infect., 2239950, PMID: 37470396, DOI: 10.1080/22221751.2023.2239950 (link)
- Del Prete, Gregory, et al. (2019). TLR7 agonist administration to SIV-infected macaques receiving early initiated cART does not induce plasma viremia. JCI Insight, 19 pgs. PMID: 31167974. (link)
- Chaudhary, Omkar, et al. (2018). Inhibition of p38MAPK in combination with ART reduces SIV-induced immune activation and provides additional protection from immune system deterioration. PLOS Pathogens, 29 pgs. PMID: 30161247. (link)
- Francica, Joseph, et al. (2017). Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs. Blood Advances, 14 pgs. PMID: 29296883. (link)
- Roques, Pierre, et al. (2017). Attenuated and vectored vaccines protect nonhuman primates against Chikungunya virus. JCI Insight, 20 pgs. PMID: 28352649. (link)
- Marzi, Andrea, et al. (2016). Efficacy of Vesicular Stomatitis Virus–Ebola Virus Postexposure Treatment in Rhesus Macaques Infected With Ebola Virus Makona. The Journal of Infectious Diseases, 7 pgs. PMID: 27496978. (link)
- Nguyen, Nguyen Van, et al. (2016). Differential Induction of Type I Interferons in Macaques by Wild-type Measles Virus or Wild-type Measles Virus with the Hemagglutinin Protein of the Edmonston Vaccine strain. Microbiology and Immunology, 5 pgs. PMID: 27278100. (link)
- Haberthur, Kristen, et al. (2014). Intrabronchial Infection of Rhesus Macaques with Simian Varicella Virus Results in a Robust Immune Response in the Lungs. JVI, 16 pgs. PMID: 25142604. (link)
- Teigler, Jeffrey (2014). Differential Innate Immune Stimulation Elicited by Adenovirus and Poxvirus Vaccine Vectors. Harvard University, 161 pgs. PMID: no PMID. (link)
- Meyer, Christine, et al. (2013). Bacterial artificial chromosome derived simian varicella virus is pathogenic in vivo. Virology, 12 pgs. PMID: 24010815. (link)
- Schramm, Lynnise, et al. (2012). High-Throughput Quantitative Real-Time Polymerase Chain Reaction Array for Absolute and Relative Quantification of Rhesus Macaque Types I, II, and III Interferon and Their Subtypes. Journal of Interferon & Cytokine Research, 9 pgs. PMID: 22817480. (link)
- Teigler, et al. (2012). Vaccination with Adenovirus Serotypes 35, 26, and 48 Elicits Higher Levels of Innate Cytokine Responses Than Adenovirus Serotype 5 in Rhesus Monkeys. JVI, 8 pgs. PMID: 22787208. (link)
- Nan, et al. (2012). Induction of Type I Interferons by a Novel Porcine Reproductive and Respiratory Syndrome Virus Isolate. Virology, 9 pgs. PMID: 22704065. (link)
- Zaidi, Mohammad (2011). Decreased Proviral DNA Loads and Changes in Innate Immune System-Related Transcription Factor Expression in Simian Immunodeficiency Virus-Infected Rhesus Macaques after Blocking the a4b7 Gut-Homing Integrin. Emory University, 62 pgs. PMID: no PMID. (no link)
Background Literature:
- Krause CD, Pestka S. (2005) “Evolution of the Class 2 cytokines and receptors, and discovery of new friends and relatives”, Pharmacol Ther. 106(3):299-346.
- Fitzgerald-Bocarsly P, Dai J, Singh S. (2008) “Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history”, Cytokine Growth Factor Rev. 19(1):3-19.
- Carlsson HE, Schapiro SJ, Farah I, Hau J (2004) “Use of primates in research: a global overview”, Am J Primatol 63:225- 237.
- Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, Sabourin PJ, Long JP, García-Sastre A, Tolnay AE, Albrecht R, Pyles JA, Olson PH, Aicher LD, Rosenzweig ER, Murali-Krishna K, Clark EA, Kotur MS, Fornek JL, Proll S, Palermo RE, Sabourin CL, Katze MG. (2009) “Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus”, Proc Natl Acad Sci. 106(9):3455-60.
- Wagner TL, Horton VL, Carlson GL, Myhre PE, Gibson SJ, Imbertson LM, Tomai MA (1997) “Induction of cytokines in cynomolgus monkeys by the immune response modifiers, imiquimod, S-27609 and S-28463”, Cytokine, 9(11):837-45.
- Kandimalla ER, Bhagat L, Li Y, Yu D, Wang D, Cong YP, Song SS, Tang JX, Sulliva T, Agrawal S. (2005) “Immunomodulatory oligonucleotides containing a cytosine-phosphate-2’-deoxy-7- deazaguanosine motif as potent toll-like receptor 9 agonists”, Proc Natl Acad Sci. 102(19):6925-30.
- ICH Guidance for Industry, S8 Immunotoxicity Studies for Human Pharmaceuticals 2006, S6 Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals 1997.
- Staehelin, T., Stahli, C., Hobbs, D.S., and Peskta, S. (1981) “A Rapid Quantitative Assay of High Sensitivity for Human Leukocyte Interferon with Monoclonal Antibodies,” in Methods in Enzymology, Vol. 79 (S. Peskta, ed.), Academic Press, New York, 589-595.
- Kelder, B., Rashidbaigi, A., and Pestka, S. (1986) “A Sandwich Radioimmunoassay for Human IFNβ,” in Methods in Enzymology, Vol. 119 (S. Pestka, ed.), Academic Press, New York, 582-587.
- Human IFN-α international reference standard provides by NIH, reference no. GXa01-901-535. Pestka (1986) “Inteferon standards and general Abbreviation,” in Methods in Enzymology, Vol. 119 (S. Pestka.ed.) Academic Press New York 14-23.
- Rubinstein, M., Levey W.P., Moschera, J.A., Lai, C.-Y., Hershberg, R.D., Bartlett, R.T., and Pestka (1981) Human Leukocyte Interferons: Isolation and Characterization of Several Molecular Forms,” Arch Biochem. Biophys. 210, 307-318.