Mouse IFN-Beta ELISA Kit, High Sensitivity (Serum, Plasma, TCM)
Catalog Number: 42410
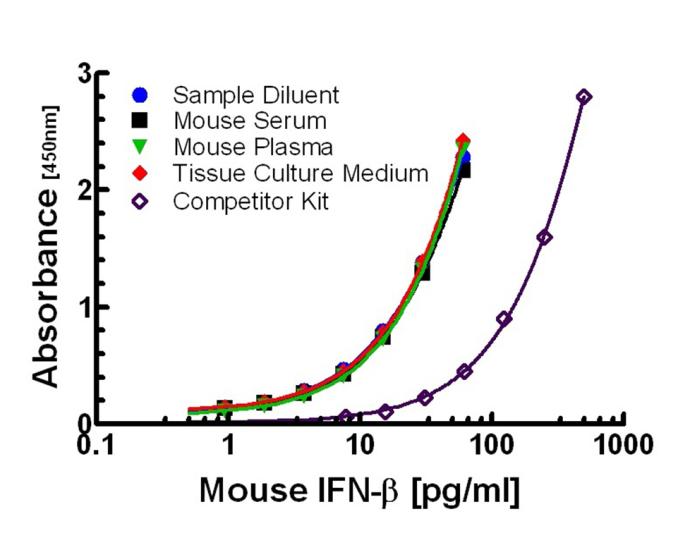
This Mouse Interferon Beta ELISA has an LLOQ of 0.94 pg/ml and is compatible with serum, plasma, and tissue cultre media (TCM).
Product Name: VeriKine-HS Mouse Interferon-Beta ELISA Kit
$595.00
$645.00
Product Info
| Matrix Compatibility | Serum, Plasma, Tissue Culture Media (TCM) |
|---|---|
| Assay Range | 0.94 - 60 pg/ml |
| LLOQ |
0.94 pg/ml Need more sensitivity? Check out our Sample Testing Services |
| Assay Length | 1 hour, 50 minutes |
| Specificity | Mouse Interferon Beta |
PBL's high sensitivity ELISA kit will equip you with the most sensitive and consistent tool for accurately measuring Mouse IFN-Beta in serum and plasma matrices. This kit quantitates Mouse Interferon Beta (IFN-Beta) in sera, plasma and tissue culture media by sandwich enzyme-linked immunosorbent assay (ELISA). Interferon binds to plates coated with antibody and detection is accomplished using a detection antibody followed by streptavidin conjugated to horseradish peroxidase (HRP). This ELISA kit utilizes Tetramethyl-benzidine (TMB) as the substrate.
Purified, recombinant mouse IFN-Beta expressed in mammalian cells is provided as the standard. The sensitivity provided by this kit, to less than 1 pg/ml in serum and plasma, will propel your mouse studies and help uncover insights into the pathology of diseases.
Specifications
| CVs and Spike Recovery |
Inter-Assay ≤ 7%
Average Spike Recovery: > 95% |
|---|---|
| Cross-reactivity |
No cross-reactivity against
|
| Synonyms | Mouse Beta Interferon, Mouse IFN Beta, Mouse Type I IFN beta, Mouse Fibroblast IFN, Mouse Fibroblast Interferon, Mouse Beta IFN, Mouse Type I Interferon Beta, Mouse IFN B |
| Storage | 2-8°C |
| Expiration Date | One year from the date of manufacture |
| Shipping Conditions | Wet Ice |
Materials Provided
- Pre-coated microtiter plate(s)
- Plate Sealers
- Wash Solution Concentrate
- Mouse Interferon Beta Standard, 10,000 pg/ml
- Sample Diluent
- Serum Buffer
- Antibody Concentrate
- Antibody Diluent
- HRP Conjugate Concentrate
- HRP Diluent
- TMB Substrate
- Stop Solution
Additional Materials Required (Not Provided)
- Microplate reader capable of reading an OD at a wavelength of 450 nm
- Variable volume microtiter pipettes
- Adjustable multichannel pipette (50-300 μl)
- Reagent reservoirs
- Wash bottle or plate washing system
- Distilled or deionized water
- Serological pipettes (1, 5, 10 or 25 ml)
- Disposable pipette tips (polypropylene)
- Plate shaker
Tech Info & Data
Intra and Inter-Assay CVs to measure Precision |
|||
| Concentration | 2.5 | 10 | 50 |
|---|---|---|---|
| Intra-Assay CV (%) | 5.7 | 5.5 | 5.5 |
| Inter-Assay CV (%) | 8.3 | 6.3 | 4.8 |
| Average Recovery (%) | 98 | 92 | 94 |
Background
The versatility of mouse models lends their use in a number of disease and autoimmunity studies. Interferons (IFNs) are a group of cytokines that exhibit pleiotropic activities that play major roles in both innate and adaptive immunity. Type I IFNs consist of multiple Interferon Alpha (IFN-α) genes and at least one Interferon Beta (IFN-β) gene in most vertebrates1,2.
Interferon Beta plays a pivotal role in the protective response to many infections and diseases3 due to its antiviral and immune-modulatory activities and is an important early product of TLR/RLR stimulation. However, when produced unchecked it can also contribute to the generation of clinically relevant side effects and pathological processes4-6. Additionally, IFN-β is a common therapeutic treatment for multiple sclerosis and some cancers with the research into these diseases often conducted in mice7.
Citations
54 Citations:
- Sales Conniff, A. et al., (2024), "Pulsed Electric Fields Induces STING Palmitoylation and Polymerization Independently of Plasmid DNA ELectrotransfer", Pharmaceutics, 16:363, DOI: 10.3390/pharmaceutics16030363 (link)
- Casella, V. et al., (2023), "Differential kinetics of splenic CD169+ macrophage death is one underlying cause of virus infection fate regulation", Cell Death Dis., 14(12):838, PMID: 38110339, DOI: 10.1038/s41419-023-06374-y (link)
- Tong et al., (2023). "Nucleotide modifications enable rational design of TLR7-selective ligands by blocking RNase cleavage", J. Exp Med., 221(2):e20230341, PMID: 38095631, DOI: 10.1084/jem.20230341 (link)
- Nilsen, K.E., et al., (2023), "Peptide derived from SLAMF1 prevents TLR4-mediated inflammation in vitro and in vivo", Life Sci Alliance, 6(12):e202302164, PMID: 37788908, DOI: 10.26508/isa.202302164 (link)
- Uccello, T.P., et al., (2023), "New insights into the responder/nonresponder divide in rectal cancer: Damage-induced Type I IFNs dictate treatment efficacy and can be targeted to enhance radiotherapy", Cell death Dis., 14(7):470, PMID: 37495596, DOI: 10.1038/s41419-023-05999-3 (link)
- Liu, H. et al., (2023), "Activated cGAS/STING signaling elicits endothelial cell senescence in early diabetic retinopathy", JCI Insight, 8(12):e168945, PMID: 37345657, DOI: 10.1171/jci.insight.168945 (link)
- Li, N. et al., (2023), "STING controls opioidinduced itch and chronic itch via spinal tank-binding kinase 1-dependent type I interferon response in mice", J. Neuroinflammation, 20(1):101, PMID: 37122031, DOI: 10.1186/s12974-023-02783-0 (link)
- Udeochu, J.C. et al., (2023), "Tau activation of microglial cGAS-IFN reduces MEF2C-mediated cognitive resilience", Nat. Neurosci., PMID: 37095396, DOI: 10.1038/s41593-023-01315-6 (link)
- Wahl, D., et al., (2023), "The reverse transcriptase inhibitor 3TC protects against age-related cognitive dysfunction", Aging Cell, e13798, PMID: 36949552, DOI: 10.1111/acel.13798 (link)
- Vornholz, L. et al., (2023), "Synthetic enforcement of STING signaling in cancer cells appropriates the immune microenvironment for checkpoint inhibitor therapy", Sci. Adv. 9(11):eadd8564, PMID: 36921054, DOI: 10.1126/sciadv.add8564 (link)
- Preston, S.P. et al., (2023), "A necroptosis-independent function of RIPK3 promotes immune dysfunction and prevents control of chronic LCMV infection", Cell Death Dis. 14(2):123, PMID: 36792599, DOI: 10.1038/s41419-023-05635-0 (link)
- Rodruguez-Ruiz, M.E. et al., (2023), "Intratumoral BO-112 in combination with radiotherapy synergizes to achieve CD8 T-cell-mediated local tumor control", J. Immunother. Cancer, 11(1):e005011, PMID: 36631161, DOI: 10.1136/jitc-2022-005011(link)
- Kwart, D. et al., (2022), "Cancer cell-derived type I interferons instruct tumor monocyte polarization", Cell Rep., 41(10):111769, PMID: 36476866, DOI:10.1016/j.celrep.2022.111769 (link)
- Chem, J. et al., (2022), "Age-induced prostaglandin E2 impairs mitochondrial fitness and increases mortality to influenza infection", Nat. Commun., 13(1):6759, PMID: 36351902, DOI: 10.1038/s41467-022-34593-y (link)
- Mansouri, S. et al., (2022), "MPYS Modulates Fatty Acid Metabolism and Immune Tolerance at Homeostasis Independent of Type I IFNs", J. Immunol., ji2200158, PMID: 36261171, DOI: 10.1049/jimmunol.2200158 (link)
- Xia, Y., et al., (2022), "TGFβ reprograms TNF stimulation of macrophages towards a non-cannonical pathway driving inflammatory osteoclastogenesis", Nat. Commun.,13(1):3920, PMID: 35798734, DOI: 10.1038/s41467-022-31475-1 (link)
- Mottis, A., et al., (2022), "Tetracycline-induced mitohormesis mediates disease tolerance against influenza", J. Clin. Invest. e:151540, PMID: 35787521, DOI: 10.1172/JCI151540 (link)
- Li, H. et al., (2022), "AXL targeting restores PD-1 blockade sensitivity of STK11/LKB1 mutant NSCLC through expansion of TCF1+ CD8 T cells". Cell Reports Medicine, 3:100554, DOI: 10.1016/j.xcrm.2022.100554 (link)
- Benoit-Lizon, I. et al., (2022), "CD4 cell-intrinsic STING signaling controls the differentiation and effector functions of TH1 and TH9 cells", Journal of Immuno Therapy Cancer, 10:e003459, DOI: 10.1136/jitc-2021-003459 (link)
- Yang, K. et al., (2021), "Suppression of local type I interferon by gut microbiota-derived butyrate impairs antitumor effects of ionizing radiation", JEM, 218(3):e20201915, PMID: 33496784, DOI: 10.1084/jem.20201915 (link)
- Baidya S et al., (2021), Dual Effect Organo - Germanium Compound THGP on RIG-I-Mediated Viral Sensing and Viral Replication during Influenza a Virus Infection, Viruses 13(9), (link)
-
Zhang et al., (2020), Type I interferon signaling mediates Mycobacterium tuberculosis-induced macrophage death, JEM 218(2), PMID: 33125053 (link)
-
Takenaka, Wataru, et al. (2020). Radiation Dose Escalation is Crucial in Anti-CTLA-4 Antibody Therapy to Enhance Local and Distant Antitumor Effect in Murine Osteosarcoma. Cancers, 15 pgs. PMID: 32545427. (link)
-
Zuo, Pengfei, et al. (2020). Protease-activated receptor 2 deficiency in hematopoietic lineage protects against myocardial infarction through attenuated inflammatory response and fibrosis. Biochemical and Biophysical Research Communications, 8 pgs. PMID: 32204916. (link)
- Mine, Keiichiro, et al. (2019). Impaired upregulation of Stat2 gene restrictive to pancreatic β-cells is responsible for virus-induced diabetes in DBA/2 mice. Biochemical and Biophysical Research Communications, 8 pgs. PMID: 31708097. (link)
- Schadt, Linda, et al. (2019). Cancer-Cell-Intrinsic cGAS Expression Mediates Tumor Immunogenicity. Cell Reports, 21 pgs. PMID: 31665636. (link)
- Miyauchi, et al. (2019). Effect of inactivated Streptococcus pneumoniae as non-pathogenic particles on the severity of pneumonia caused by respiratory syncytial virus infection in mice. Toxicology Reports, 27 pgs. PMID: 31245279. (link)
- Kim, et al. (2019). Small Heterodimer Partner Controls the Virus-Mediated Antiviral Immune Response by Targeting CREB-Binding Protein in the Nucleus. Cell Reports, 20 pgs. PMID: 31091449. (link)
- Pang, Zheng (2019). Regulation of Inflammation During Pseudomonas Aeruginosa Lung Infection. Dalhousie University, 177 pgs. PMID: no PMID. (link)
- Kim, Sung Phil, et al. (2018). The composition of a bioprocessed shiitake (Lentinus edodes) mushroom mycelia and rice bran formulation and its antimicrobial effects against Salmonella enterica subsp. enterica serovar Typhimurium strain SL1344 in macrophage cells and in mice. BMC Complementary and Alternative Medicine, 12 pgs. PMID: 30518352. (link)
- Zhang, Lele, et al. (2018). The deubiquitinase CYLD is a specific checkpoint of the STING antiviral signaling pathway. PLOS Pathogens, 23 pgs. PMID: 30388174. (link)
- Inoue, Kazuki, et al. (2018). Bone protection by inhibition of microRNA-182. Nature Communications, 17 pgs. PMID: 30291236. (link)
- Chaudhary, Omkar, et al. (2018). Inhibition of p38MAPK in combination with ART reduces SIV-induced immune activation and provides additional protection from immune system deterioration. PLOS Pathogens, 29 pgs. PMID: 30161247. (link)
- Rossi, Matteo, et al. (2017). Type I interferons induced by endogenous or exogenous viral infections promote metastasis and relapse of leishmaniasis. PNAS, 6 pgs. PMID: 28439019. (link)
- Vermillion, et al. (2017). Intrauterine Zika Virus Infection of Pregnant Immunocompetent Mice Models Transplacental Transmission and Adverse Perinatal Outcomes. Nature Communications, 14 pgs. PMID: 28220786. (link)
- Yamada, Taisho, et al. (2017). FICZ Exposure and Viral Infection in Mice. Bio-Protocol. PMID: no PMID. (link)
- Ahlers, et al. (2016). Invertebrate Iridescent Virus 6, a DNA Virus, Stimulates a Mammalian Innate Immune Response Through RIG-I-Like Receptors. PLOS One, 21 pgs. PMID: 27824940. (link)
- Vitner, et al. (2016). Induction of the Type I Interferon Response in Neurological Forms of Gaucher Disease. Journal of Neuroinflammation, 15 pgs. PMID: 27175482. (link)
- Ranoa, et al. (2016). Cancer Therapies Activate RIG-I-Like Receptor Pathway Through Endogenous Non-Coding RNAs. Oncotarget, 20 pgs. PMID: 27034163. (link)
- Fukui, Ryutaro, et al. (2016). Type I IFN Contributes to the Phenotype of Unc93b1D34A/D34A Mice by Regulating TLR7 Expression in B Cells and Dendritic Cells. Journal of Immunology, 13 pgs. PMID: 26621862. (link)
- Earl, Patricia, et al. (2016). Genetic Studies of the Susceptibility of Classical and Wild-Derived Inbred Mouse Strains to Monkeypox Virus. Virology, 11 pgs. PMID: 25791934. (link)
- Chen, Hao Hang Rachel (2016). The effect of pandemic influenza H1N1 viral infection on house dust mite sensitized mice. University of British Columbia, 87 pgs. PMID: no PMID. (link)
- Hoang, Hang Thi Thu (2016). Analysis of host genetic factors influencing susceptibility to influenza A infections using knockout mice. University of Veterinary Medicine Hannover, 137 pgs. PMID: no PMID. (no link)
- Rivera-Toledo, Evelyn, et al. (2015). Respiratory Syncytial Virus Persistence in Murine Macrophages Impairs IFN-β Response but Not Synthesis. Viruses, 14 pgs. PMID: 26501312. (link)
- Cui, et al. (2015). Viral DNA-Dependent Induction of Innate Immune Response to Hepatitis B Virus in Immortalized Mouse Hepatocytes. JVI, 11 pgs. PMID: 26491170. (link)
- Hargadon, Kristian, et al. (2015). Melanoma-derived factors alter the maturation and activation of differentiated tissue-resident dendritic cells. Immunology and Cell Biology, 15 pgs. PMID: 26010746. (link)
- Hanson, Melissa, et al. (2015). Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. JCI, 15 pgs. PMID: 25938786. (link)
- Detje, Claudia, et al. (2015). Upon Intranasal Vesicular Stomatitis Virus Infection, Astrocytes in the Olfactory Bulb Are Important Interferon Beta Producers That Protect from Lethal Encephalitis. JVI, 8 pgs. PMID: 25540366. (link)
- White, Michael, et al. (2014). Apoptotic Caspases Suppress mtDNA-Induced STING-Mediated Type I IFN Production. CellPress, 27 pgs. PMID: 25525874. (link)
- Deng, Liufu, et al. (2014). STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity, 22 pgs. PMID: 25517616. (link)
- Lalani, Almin, et al. (2014). Myeloid Cell TRAF3 Regulates Immune Responses and Inhibits Inflammation and Tumor Development in Mice. Journal of Immunology, 36 pgs. PMID: 25422508. (link)
- O'Reilly, L. A., et al. (2014). Loss of c-REL but not NF-κB2 prevents autoimmune disease driven by FasL mutation. Cell Death & Differentiation, 12 pgs. PMID: 25361085. (link)
- Wolferstaetter, Michael, et al. (2014). Recombinant Modified Vaccinia Virus Ankara Generating Excess Early Double-Stranded RNA Transiently Activates Protein Kinase R and Triggers Enhanced Innate Immune Responses. JVI, 16 pgs. PMID: 25297997. (link)
- Rosenberger, Catherine, et al. (2014). Characterization of innate responses to influenza virus infection in a novel lung type I epithelial cell model. Journal of General Virology, 13 pgs. PMID: 24243730. (link)
Background Literature:
- A Rapid Quantitative Assay of High Sensitivity for Human Leukocyte Interferon with Monoclonal Antibodies. Staehelin et al., 1981, Methods in Enzymology (S. Peskta, ed.). 79: 589-595.
- A FADD-dependent innate immune mechanism in mammalian cells. Balachandran et al., 2004, Nature. 432: 401-405.