Human IFN-Alpha All Subtype ELISA Kit, High Sensitivity (TCM)
Catalog Number: 41135
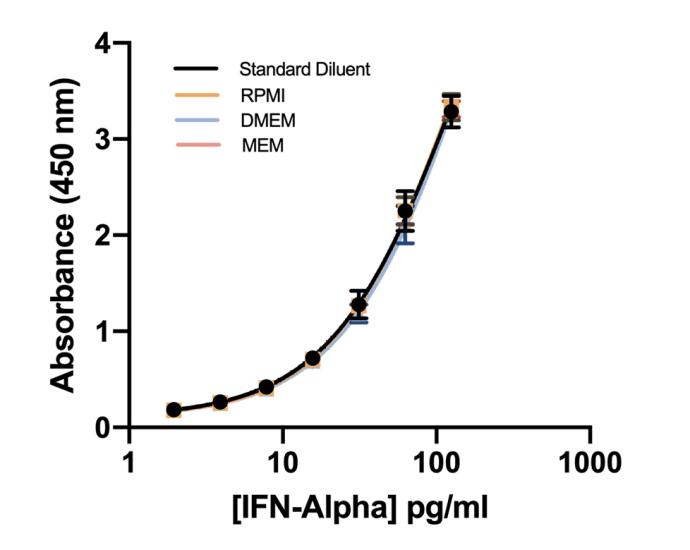
This ELISA quantitates the amount of IFN-Alpha in tissue culture media (TCM) with an LLOQ of 1.95 pg/ml. This kit provides sensitive and total detection of all human IFN-Alpha subtypes.
Product Name: VeriKine-HS Human Interferon-Alpha All-Subtype ELISA Kit (TCM)
$545.00
$645.00
Product Info
| Matrix Compatibility | Tissue Culture Media (TCM) |
|---|---|
| Assay Range | 1.95 - 125 pg/ml |
| LLOQ | 1.95 pg/ml Need more sensitivity? Check out our Sample Testing Services |
| Assay Length | 20 hours 30 minutes - 24 hours 30 minutes |
| Specificity | Human IFN-α |
The VeriKine-HS Human Interferon Alpha All Subtype TCM ELISA Kit is specifically formulated to detect all human IFN-Alpha subtypes. This kit quantitates all human IFN alpha subtypes in tissue culture media (TCM) using a sandwich immunoassay format. The kit is based on an ELISA with biotinylated-detection antibody and streptavidin-conjugated horseradish peroxidase (HRP). Tetramethyl-benzidine (TMB) is the substrate. The assay is based on the international reference standard for human interferon alpha provided by the National Institutes of Health.
*For complex biological matrices such as plasma, healthy sera, and autoimmune disease sera, we recommend our Human IFN-Alpha All Subtype ELISA Kit, High Sensitivity (Cat. No. 41115-1).
Specifications
CVs and Spike Recovery | Inter-Assay: ≤ 10%
|
|---|---|
Cross-reactivity | Cross-reacts with
No cross-reactivity against
|
Storage | 2-8°C |
Expiration Date | One year from the date of manufacture |
Shipping Condition | Wet Ice |
Materials Provided
- Pre-coated microtiter plate
- Plate Sealers
- Wash Solution Concentrate
- Human Interferon Alpha Standard, 10,000 pg/ml
- Assay Buffer
- Standard Diluent
- Antibody Diluent
- Antibody Concentrate
- HRP Conjugate Concentrate
- HRP Diluent
- TMB Substrate Solution
- Stop Solution
Additional Materials Required (Not Provided)
- Microtiter plate reader capable of reading a wavelength of 450 nm
- Plate shaker (recommended shaking capability of 550 rpm)
- Variable volume microtiter pipettes
- Adjustable multi-channel pipette (50-300 μl)
- Reagent reservoirs
- Wash bottle or plate washing system
- Distilled or deionized water
- Serological pipettes (1, 5, 10 or 25 ml)
- Disposable pipette tips (polypropylene)
Tech Info & Data
Tips, Tools and Troubleshooting:
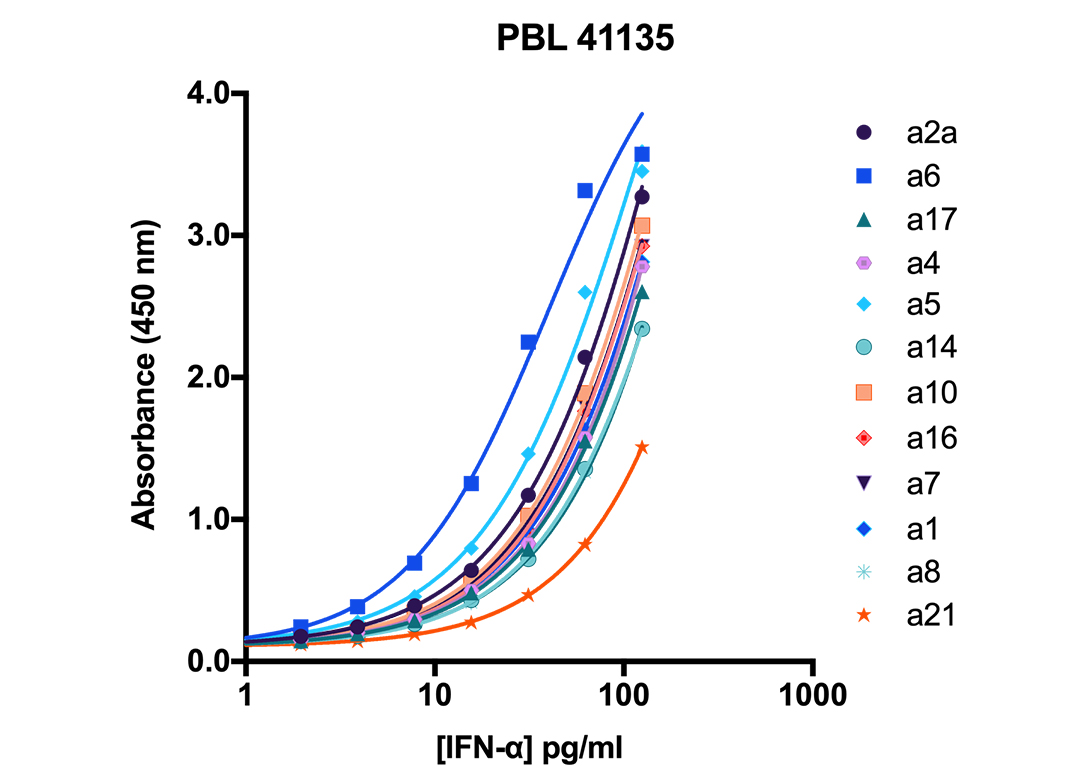
| Subtype | LLOQ |
|---|---|
Representative Human IFN-Alpha All Subtype Detection | |
| α1 (αD) | 2.06 |
| α2a (αA) | 1.41 |
| α4a (αM1) | 2.03 |
| α5 (αG) | 1.20 |
| α6 (αK) | 0.82 |
| α7 (αJ1) | 1.93 |
| α8 (αB2) | 2.67 |
| α10 (αC) | 1.98 |
| α14 (αH) | 3.06 |
| α16 (αWA) | 2.39 |
| α17 (αI) | 2.26 |
| α21 (αF) | 4.96 |
Representative Standard Curves of Human IFN-Alpha Subtypes
Intra-Assay Precision | |||
|---|---|---|---|
| TC Media Spike Sample | 1 | 2 | 3 |
| n | 20 | 20 | 20 |
| Mean (pg/ml) | 2.39 | 12.90 | 87.16 |
| Standard Deviation | 0.13 | 0.46 | 4.58 |
| CV (%) | 5.4 | 3.6 | 5.3 |
Inter-Assay Precision | |||
|---|---|---|---|
| TC Media Spike Sample | 1 | 2 | 3 |
| n | 9 | 9 | 9 |
| Mean (pg/ml) | 3.29 | 15.13 | 93.69 |
| Standard Deviation | 0.16 | 0.83 | 6.07 |
| CV (%) | 4.9 | 5.5 | 6.5 |
Intermediate Precision | |||
|---|---|---|---|
| TC Media Spike Sample | 1 | 2 | 3 |
| n | 9 | 9 | 9 |
| Mean (pg/ml) | 3.29 | 15.30 | 94.00 |
| Standard Deviation | 0.44 | 1.75 | 7.37 |
| CV (%) | 13.4 | 11.4 | 7.8 |
Spike Recovery | |||
|---|---|---|---|
| TC Media Spike Sample | 1 | 2 | 3 |
| n | 25 | 25 | 25 |
| Average Recovery (%) | 109 | 102 | 104 |
| Range | 86-143 | 81-122 | 90-120 |
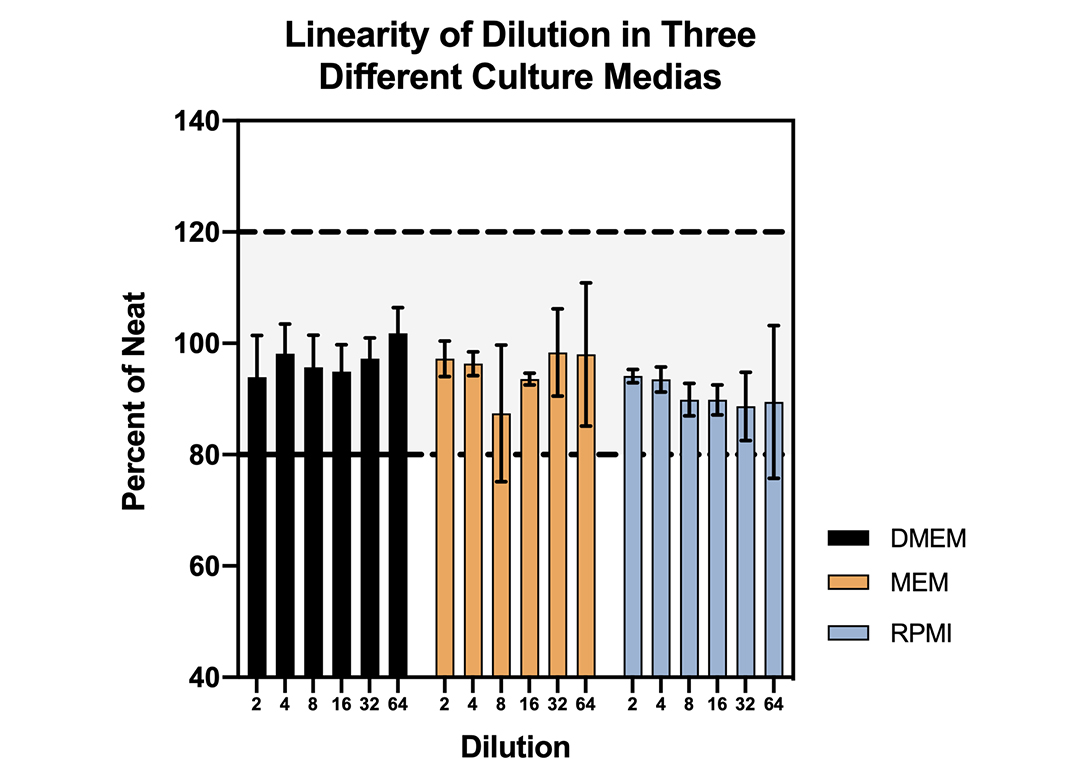
Linearity
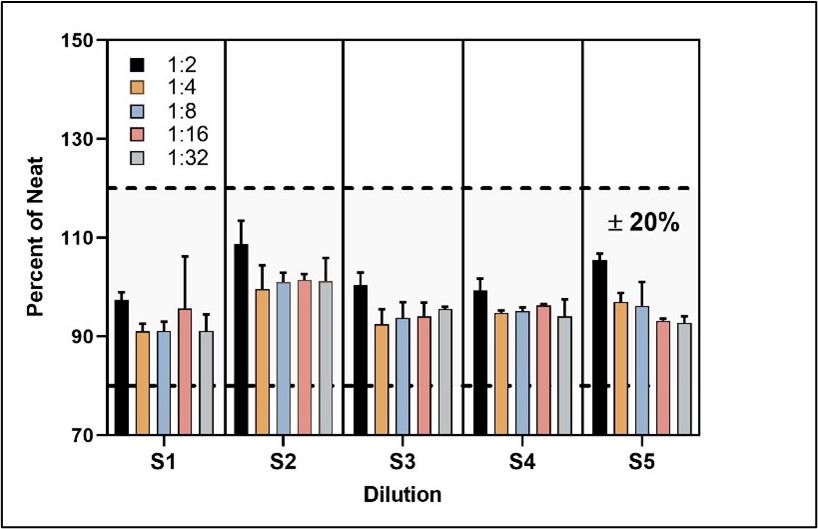
Parallelism of Endogenous Samples
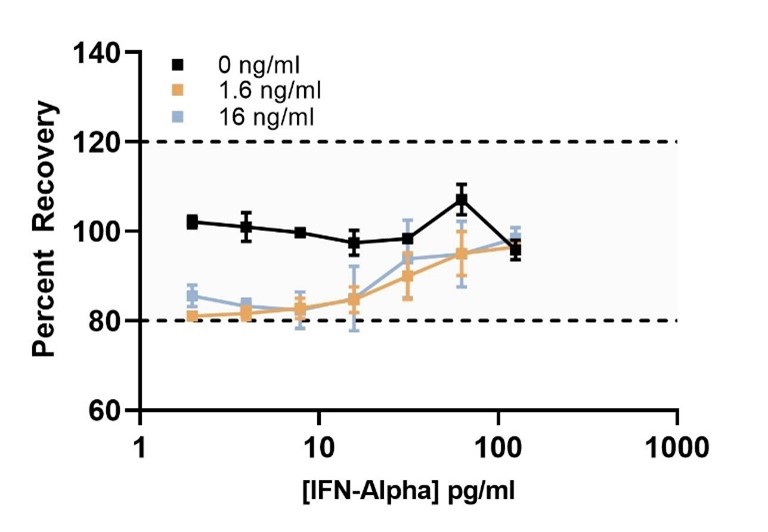
sIFNAR2 Receptor Interference
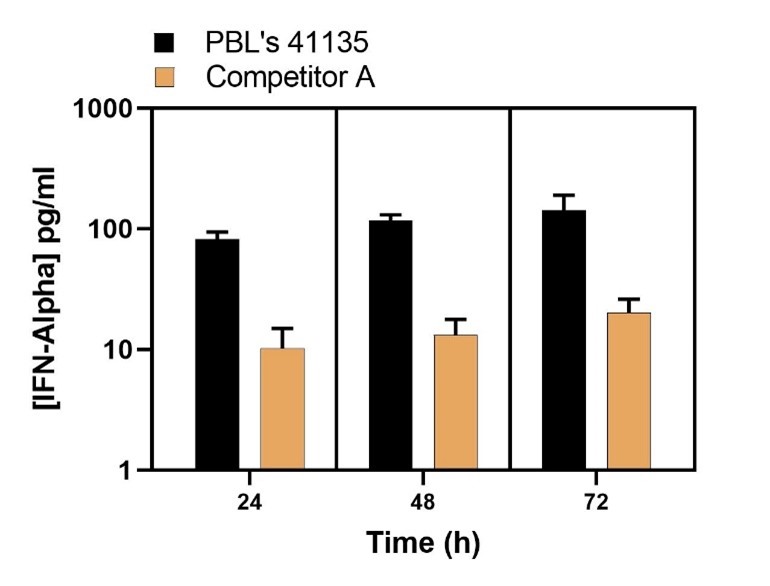
Endogenous Levels of IFN-Alpha Quantified in Sendai Virus (A549)
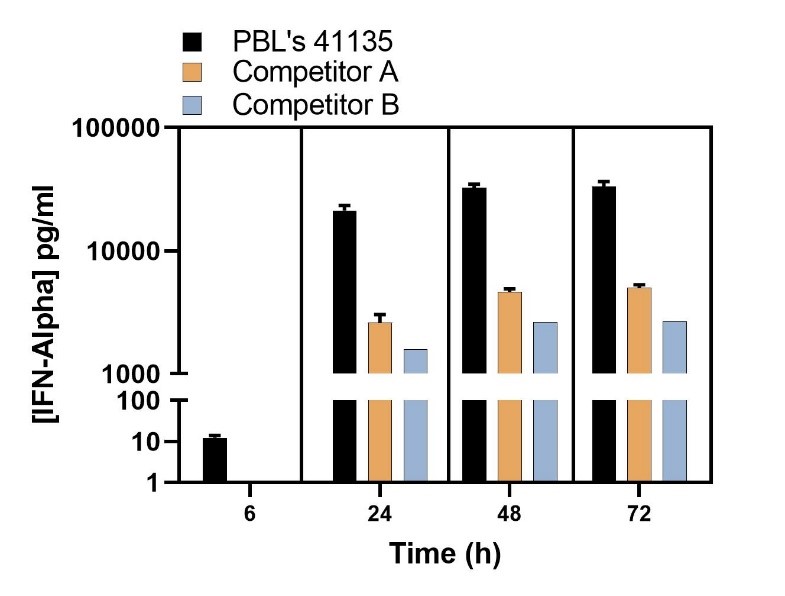
Endogenous Levels of IFN-Alpha Quantified in Sendai Virus (U937)
Background
Interferons (IFNs) are a family of mammalian cytokines initially characterized by their ability to inhibit viral infection. They are synthesized and secreted by most cell types in response to pathogens. In addition to their antiviral properties, IFNs have also been shown to exhibit anti-proliferative, immunomodulatory, and many other activities.
In humans, IFN-α refers to a family of proteins comprised of 12 highly homologous protein subtypes (greater than 85% by amino acid sequence), encoded by 13 genes, that exhibit pleiotropic biologic activities. Measuring the levels of the 12 different human IFN-α subtypes is essential for understanding their triggers and subsequent effects on the immune system. The ability to measure all the subtypes may provide an indication of the total IFN-α level.
Citations
9 Citations:
- Bulut, O. et al., (2024), "Alendronate Modulates Cytokine Responses in Healthy Young Individuals After BCG Vaccination", Immunol Lett., PMID: 38479480, DOi: 10.1016/j.imlet.2024.106851 (link)
- Salzmann, R.J.S. et al., (2024), "Increased type-I interferon level is associated with liver damage and fibrosis in primary sclerosing cholangitis", Hepatol Commun., 8(3):e0380, PIMD: 38358371, DOI: 10.1097/HC9.0000000000000380, (link)
- Roring RJ. et al., (2024), "MMR vaccination induces trained immunity via functional and metabolic reprogramming of γδ T cells", J. Clin Invest. e170848, PMID: 38290093, DOI: 10.1172/JCI170848 (link)
- Heider, A. et al., (2024), "Characteristics of two zoonotic swine influenza A(H1N1) viruses isolated in Germany from diseased patients", International Journal of Medical Microbiology, 314:151609, DOI: 10.1016/j.ijmm.2024.151609 (link)
- Ide, M. et al., (2024), "Hepatitis B virus evades the immune system by suppressing the NF-kB signaling pathway with DENND2A", Microbiol Spectr., e0378523, PMID: 38240571, DOI: 10.1128/spectrum.03785-23 (link)
- Luganini, A., et al., (2023), "Mechanisms of antiviral activity of the new hDHODH inhibitor MEDS433 against respiratory syncytial virus replication", Antiviral Res., 105734, PMID: 37852322, DOI: 10.1016/j.antiviral.2023.105734 (link)
- Sugimoto, A. et al., (2023), "Identification of novel lactic acid bacteria with enhanced protective effects against influenza virus", PLoS One, 18(8):e0273604, PMID: 37556447, DOI: 10.1371/journal.pone.0273604 (link)
- Killarney, S. et al., (2023), "Executioner caspases restrict mitochondrial RNA-driven Type I IFN induction during chemotherapy-induced apoptosis", Nat. Commun. 14(1):1399, PMID: 36918588, DOI: 10.1038/s41467-023-37146-z (link)
- Dowling, D.J. et al., (2022), "development of a TLR7/8 agonist adjuvant formulation to overcome early life hyporesponsiveness to DTaP vaccination", Sci. Rep. 12(1):16860, PMID: 36258023, DOI: 10.1038/s41598-022-20346-w (link)
Background Literature:
- Staehelin et al. (1981) "A Rapid Quantitative Assay of High Sensitivity for Human Leukocyte Interferon with Monoclonal Antibodies" in Methods in Enzymology, Vol. 79 (Pestka, ed.), Academic Press, New York, 589-595.
- Kelder et al. (1986) "A Sandwich Radioimmunoassay for Human IFNa" in Methods in Enzymology, Vol. 119 (Pestka, ed.), Academic Press, New York, 582-587.
- Human IFN-α international reference standard provided by the NIH, reference no. Gxa01-901-535. Pestka (1986) "Interferon Standards and General Abbreviations" in Methods in Enzymology, Vol. 119 (Pestka, ed.), Academic Press, New York, 14-23.
- Rubinstein et al. (1981) "Human Leukocyte Interferon: Isolation and Characterization of Several Molecular Forms," Arch. Biochem. Biophys. 210, 307-318.
- Hobbs et al. (1982) "Purification and Characterization of Interferons from a Continuous Myeloblastic Cell Line," J. Biol. Chem. 257, 4071-4076.